Abstract
A high-temperature conductive silver slurry containing lead-free ZnO-B2O3-SiO2 glass for metallization of ceramic MgTiO3 substrate has been prepared. The ZnO-B2O3-SiO2 glass was obtained by high-temperature melting and cold extraction with a transition temperature (Tg) of 579 °C and thermal stability (∆T) of 105 °C as an inorganic bonding phase of high-temperature conductive silver paste. Then, silver paste with different glass powder content was sintered on ceramic MgTiO3 substrate between 730 °C and 930 °C in increments of 50 °C. Characterization of the prepared materials revealed that their resistivity increases with the increase in glass powder content and decreases with the increase in sintering temperature. Sintering temperature and glass content have significant effects on the resistivity and adhesion of the thick silver film. When the silver paste with a glass content of 1.1 wt% was sintered at 830 °C for 10 min, the resulting thick silver film had a low resistivity of 1.81 μΩ·cm (1.65 μΩ·cm for silver) and a good adhesive strength of 39.4 N mm−2. During the sintering process, the glass material melts and wets the silver powder, which promotes the sintering of the silver powder to form a dense network structure and improves the electrical conductivity of the silver film. In addition, the formation of ZnTiO3 by chemical reactions between the glass and the substrate was observed, which dramatically improved the bonding strength of the silver film. Therefore, lead-free silver paste containing ZnO-B2O3-SiO2 glass powder and MgTiO3 ceramics has broad development prospects in ceramic filters.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
With the rapid development of the 5G era of mobile communication and satellite communication technology, microwave dielectric ceramics as a new type of functional ceramic material developed rapidly in recent years. These ceramics exhibit low dielectric losses or high quality factors, high dielectric constants, and temperature-stable dielectric coefficients. This material has been widely used in filters, radio frequency components, and microwaves [1–5]. The filter is the core RF device of the mobile communication base station. 5G base station filters reflect the current development trend of multi-functional, compound-based, and intelligent electronic components with high frequency, thin dimensions, and low power consumption [6, 7]. MgTiO3 ceramics have certain advantages such as high quality factor (Q × ƒ = 360,000), high dielectric constant (>15), low dielectric loss (<5 × 10−5), good thermal conductivity and chemical stability, abundant material base, and low costs [8, 9]. The filter metallizes the MgTiO3 ceramic and welds it on the circuit board. After high DC voltage polarization, the filter possesses a piezoelectric effect to realize the filtering function [10]. The main methods for metalizing MgTiO3 ceramics are as follows: molybdenum and manganese metallization [11], thin film metallization [12], direct application of copper metallization [13, 14], and thick film metallization [15]. The thick film metallization method has been widely used. This method has the advantages of a simple process, low costs, and the ability to achieve high-density packaging of integrated circuits [16].
High-temperature conductive silver paste is mainly composed of conductive metal, adhesive phase powder, and organic auxiliaries [17–19]. The conductive metal is the main component of the high-temperature conductive silver paste and determines the electrical conductivity. The content is generally about 90%, and the commonly used conductive metal phases are silver, copper, aluminum, and gold [20]. The bonding phase powder is usually glass powder, which significantly influences the electrical and mechanical properties of the silver film. In the sintering process, glass powder can not only promote the sintering of silver particles to form a dense network structure but also affect the adhesion between the silver film and the MgTiO3 substrate [21, 22]. Organic additives (ethyl cellulose, diethylene glycol butyl ether) are the primary carriers of the conductive and bonding phases and can control the rheological properties, viscosity, and screen-printing performance of the slurry [23–25]. Although lead can reduce the glass transition temperature of glass powder, it can improve the performance of the silver film. However, lead-containing glass powder will cause harm to the human body and environment, for which reason it should be replaced by lead-free glass powder [26, 27].
Considerable research has been conducted on slurries prepared with different conductive metals (silver, copper, and aluminum) and different substrate materials. Yang et al (2019) prepared a lead-free Bi2O3-B2O3-ZnO glass powder with a low glass transition temperature (Tg = 360 °C) for the metallization of Al2O3 substrate; using silver paste with a glass content of 7 wt% and sintering at 600 °C, the obtained material exhibited a low resistivity of 2.5 μΩ·cm and a high adhesive strength of 28.5 MPa [28]. Sun et al (2020) reported a glass powder of P2O5-V2O5-ZnO with a Tg of 387 °C and a thermal expansion coefficient of 6.48 × 10−6/°C. The thick silver film was formed on the Al2O3 substrate by the steel-printing method and then sintered at 650 °C. The silver paste with a glass content of 1 wt% had a low resistivity of 3.13 μΩ·cm and a high shear strength of 40.47 MPa [29]. Zou et al (2021) printed a novel CuO-doped silver paste on an AlN ceramic substrate that has high adhesive strength on an AlN substrate. The 3 wt% CuO-doped thick silver film sintered at 850 °C showed excellent conductivity (0.9 mΩ/□) and adhesive strength (15.3 MPa) [16]. However, the effect of the glass material on the properties of the silver paste was not reported. Since the silver film is expected to have better electrical conductivity and adhesion properties in actual production, it is necessary to prepare different systems of glass materials to improve the performance of silver paste combined with different substrates. Although the above studies on different glass systems and ceramic substrates provide helpful guidance for this study, there are no reports on lead-free ZnO-B2O3-SiO2 glass powder as the bonding phase of conductive silver paste and MgTiO3 ceramics as the substrate.
In this work, ZnO-B2O3-SiO2 glass was prepared by high-temperature melting and cold extraction. The characteristic temperatures and amorphous properties of the glass were studied by thermogravimetry-differential scanning calorimetry (TG-DSC) and x-ray diffraction (XRD). The ZnO-B2O3-SiO2 glass acted as a bonding phase in the silver paste and was printed on the MgTiO3 ceramic substrate by screen printing followed by sintering under different conditions. The effects of sintering temperature and glass content on the conductivity and adhesion of the thick silver film were systematically investigated. The surface microstructure of the silver film and the microstructure at the interface between the silver film and substrate were also studied.
2. Experimental scheme
2.1. Materials
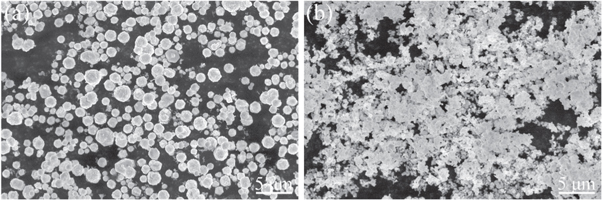
The raw materials for the glass powder were mainly purchased from Aladdin, Sinophosphoric Chemical Reagent, and West Asia Reagent and were analytically pure (AR). These materials include ZnO, B2O3, SiO2, Al2O3, Na2CO3, BaO, CaO, and K2CO3.The organic carrier consisted of ethyl cellulose (Dow) and diethylene glycol butyl ether (purity ≥ 98%, Aladdin). The MgTiO3 ceramic substrate was cut into 20 mm × 20 mm square pieces, which were ultrasonically cleaned with acetone and alcohol for 5 min each for later use. Spherical silver Ag-1 (as shown in figure 1(a)) with an average particle size of about 1 μm and irregular silver Ag-2 (as shown in figure 1(b)) were used as conductive metal phases in the silver paste, and 200-mesh screens were used for printing.
Figure 1. SEM images of silver powders. (a) Spherical silver powder Ag-1; (b) irregular silver powder Ag-2.
Download figure:
Standard image High-resolution image2.2. Preparation process of glass powder
The formulations of all glass powders in this work are presented in table 1. The formulations varied in ZnO content and were named G1, G2, G3, G4, and G5. In the preparation process of the glass powders, various oxides were accurately weighed in an agate ball mill tank according to the formula of the glass powder, and anhydrous ethanol and agate beads were added before mixing to obtain various evenly mixed oxides after drying for later use. The dried powder mixture was transferred into an Al2O3 crucible, which was placed into a muffle furnace. At a heating rate of 10 °C min−1, the mixture was heated to 1300 °C, kept at this temperature for 2 h, and then poured into deionized water for quenching. The obtained glass slag, agate beads, and anhydrous ethanol in a particular proportion were added to a planetary ball mill and ground for 21 h at 300 r min−1. The dry material was passed through 400-mesh screens to obtain the required glass powder.
Table 1. Composition of glass material (wt%).
| Sample | ZnO | B2O3 | SiO2 | Al2O3 | Na2O | BaO | CaO | K2O |
|---|---|---|---|---|---|---|---|---|
| G1 | 35 | 40 | 10 | 2 | 3 | 3 | 3 | 4 |
| G2 | 40 | 35 | 10 | 2 | 3 | 3 | 3 | 4 |
| G3 | 45 | 30 | 10 | 2 | 3 | 3 | 3 | 4 |
| G4 | 50 | 25 | 10 | 2 | 3 | 3 | 3 | 4 |
| G5 | 55 | 20 | 10 | 2 | 3 | 3 | 3 | 4 |
2.3. Preparation of silver paste and metallization of ceramics
The silver slurry was composed of silver particles, glass powder, and an organic carrier. In this work, the organic carrier consisted of ethyl cellulose (20 wt%) and diethylene glycol butyl ether (80 wt%), which were mixed according to the required proportion and reacted in a water bath at 80 °C until the ethyl cellulose was completely dissolved, followed by cooling to room temperature. The obtained organic carrier was mixed with silver powder and glass powder to prepare the silver paste. A three-roll grinder was used to distribute the particles evenly in the slurry. The composition of the silver paste in this work is shown in table 2. The thick film was prepared on the MgTiO3 substrate by screen printing followed by drying in an oven at 150 °C for 10 min. The cured sample was placed into a muffle furnace and sintered for 10 min at 730 °C, 780 °C, 830 °C, 880 °C, or 930 °C to realize the metallization of the ceramic substrate surface.
Table 2. Composition of conductive silver paste.
| Composition (wt%) | Ag-1 | Ag-2 | Glass | Bi2O3 | Cu O | Li2CO3 | Organic vehicle |
|---|---|---|---|---|---|---|---|
| A1 | 61.2 | 20 | 0.3 | 0.75 | 0.3 | 0.15 | 17.3 |
| A2 | 60.8 | 20 | 0.7 | 0.75 | 0.3 | 0.15 | 17.3 |
| A3 | 60.4 | 20 | 1.1 | 0.75 | 0.3 | 0.15 | 17.3 |
| A4 | 60.0 | 20 | 1.5 | 0.75 | 0.3 | 0.15 | 17.3 |
| A5 | 59.6 | 20 | 1.9 | 0.75 | 0.3 | 0.15 | 17.3 |
| A6 | 59.2 | 20 | 2.3 | 0.75 | 0.3 | 0.15 | 17.3 |
2.4. Characterization method
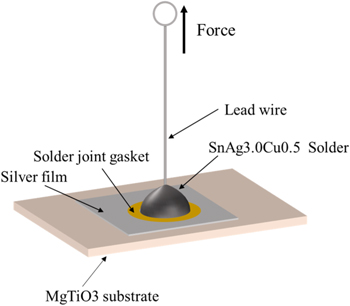
The characteristic temperatures of glass powders, such as the glass transition temperature (Tg) and crystallization temperature (Tc), were determined by TG-DSC with an STA 6000 instrument (PerkinElmer, USA). XRD was used to study the crystallization degree of glass powders to assess the quality of the glass. The electrical properties of the thick silver films were determined by a multifunctional four-point probe tester (ST-2258C, Suzhou Lattice Electronics, China). Their thicknesses were measured by a plating thickness measuring instrument (Thick 800A, Detech System PTE Ltd, Singapore). Scanning electron microscopy (SEM, SU8010, Hitachi, Japan) and energy dispersive spectroscopy (EDS) were used to observe the surface morphology of silver particles, glass powders, thick silver films, and the cross-section microstructures between thick silver films and substrates. The schematic diagram of the adhesion test between the thick silver films and substrates is shown in figure 2. The gasket with a fixed solder joint size (solder joint is a circle with a diameter of 2 mm) was pasted on the silver film, and a lead was welded on the silver film with solder (SnAg3.0Cu0.5) at 250 °C using a lead-free welding table (QUICK 203H, Quick Electronic Equipment Co., Ltd, China). A digital-display push tension meter (VICTOR 300N, Shenzhen Yisheng Shengli Technology Co., Ltd, China) was used to uniformly pull leads from the substrate to test the adhesion of the thick silver film on the MgTiO3 ceramic substrate.
Figure 2. Adhesion test diagram of thick silver film.
Download figure:
Standard image High-resolution image3. Results and discussion
3.1. Characterization of glass powder
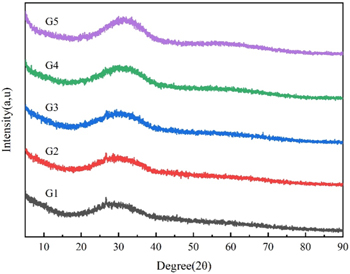
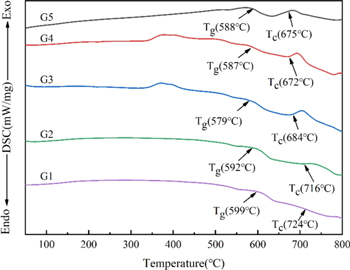
The XRD results of all glass powders are shown in figure 3. Several samples exhibited a broad peak near 2θ = 29°, the amorphous content was relatively high, and the glass-forming property was relatively good. Figure 4 shows the DSC results of the samples, from which the characteristic temperatures of the samples were obtained. As shown in table 3, the glass transition temperature Tg of the glass powders first decreased and then increased with the increase in ZnO content. When the ZnO content increased from 35% to 45%, Tg decreased from 599 °C to 579 °C. The thermal performance of glass is closely related to the degree of glass structure polymerization and the change in the quantity of [SiO4], [BO3], [BO4], [ZnO4], and [ZnO6] [30–32]. In the ZnO-B2O3-SiO2 system, ZnO is a network intermediate of glass. After capturing and supplying free oxygen, ZnO can exist in glass in the form of [ZnO4] and [ZnO6]. Because of its high field strength, ZnO has the ability to capture free oxygen. When ZnO content is less than 45%, ZnO mainly exists in the network gap as a network body due to the effect of network breaking, resulting in the generation of more 'non-bridging oxygen', the silicon-oxygen network polymerization degree decreases so that the Si-O-Si or Si-O bond force constants decrease, the structure of the glass is looser, and the fluidity of the glass is improved, resulting in a low glass transition temperature. As the ZnO content continues to increase, more Zn2+ gradually forms [ZnO4] and is involved in the internal network structure of the glass, stabilizing the internal structure; the network structure is strengthened, and the result is a high glass transition temperature. Combined with the characteristic temperature of the sample, we chose the G3 sample with a low Tg of 579 °C and high stability (ΔT = 105 °C) as the inorganic binder for the high-temperature silver paste.
Figure 3. XRD pattern of the glass sample.
Download figure:
Standard image High-resolution imageFigure 4. DSC curve of glass sample.
Download figure:
Standard image High-resolution imageTable 3. The characteristic temperature of the glass sample.
| Sample | Tg (°C) | Tc (°C) | Δ = Tc-Tg (°C) |
|---|---|---|---|
| G1 | 599 | 724 | 125 |
| G2 | 592 | 716 | 124 |
| G3 | 579 | 684 | 105 |
| G4 | 587 | 672 | 85 |
| G5 | 588 | 675 | 87 |
3.2. Influence of glass powder content on conductivity and adhesion of the silver film
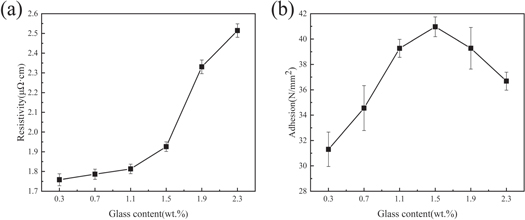
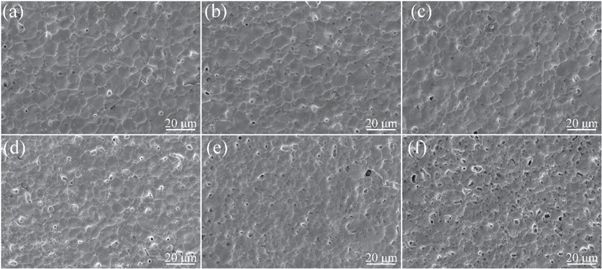
The content of glass material significantly influences the performance of silver films after sintering. Figure 5 shows the influence of the glass powder content on the conductivity and adhesion of the silver film under the same sintering conditions. According to figure 5, the resistivity of the silver film increased from 1.76 μΩ·cm to 2.51 μΩ·cm with the increase in glass content. Figure 6 shows the SEM images of the thick silver films sintered at 830 °C for 10 min with different glass powder contents, which indicate the change in film density and the decrease in grain size. With the increase in glass powder content, more glass exists on the surface of the silver film, which increases the gap between silver particles, obstructs the connection, hinders the formation of a sintering neck between silver particles, reduces the number of conductive paths inside the silver film, and thus increases the resistivity of the silver film.
Figure 5. (a) Resistivity and (b) Adhesion of silver films sintered at 830 °C with different glass contents.
Download figure:
Standard image High-resolution imageFigure 6. Surface SEM images of silver films with different glass contents sintered at 830 °C for 10 min (a) 0.3 wt% glass, (b) 0.7 wt% glass, (c) 1.1 wt% glass, (d) 1.5 wt% glass, (e) 1.9 wt% glass and (f) 2.3 wt% glass.
Download figure:
Standard image High-resolution imageAccording to figure 5(b), when the glass powder content increased from 0.3 wt% to 2.3 wt%, the adhesive strength between silver film and substrate first increased and then decreased from 31.4 N mm−2 over 34.4 N mm−2, 39.4 N mm−2, 40.9 N mm−2, and 39.2 N mm−2 to 36.8 N/mm2. The decrease in adhesion could be explained as follows. First, when the glass powder content is high, the silver layer separates from the substrate at the interface of the glass and silver layer; second, when the content of glass powder exceeds 1.5 wt%, more glass is present on the surface of the silver film, and the wettability between the glass and the solder is poor, which results in poor contact between the solder and the silver powder and, consequently, in the observed decrease in adhesion. Based on the above points, the sintered silver film with a glass content of 1.1 wt% has a lower resistivity of 1.81 μΩ·cm and a higher adhesive strength of 39.4 N mm−2.
Table 4 compares the electrical conductivity and adhesion properties of conductive silver paste prepared from silver powder as conductive phase combined with different substrates. Marcinkowski et al studied the sintering kinetics and interface formation of silver films on AlN ceramics. The conductivity of silver films prepared at 850 °C was as low as 0.93 mΩ/□, but the adhesive strength could only reach 6.7 MPa [33]. Zou et al prepared a conductive silver slurry with a solid content of up to 90% at 850 °C, resulting in a thick silver film with a block resistance of 0.9 mΩ/□ but a not particularly high adhesive strength of 15.3 MPa [16]. In this work, high-temperature silver paste with solid contents as low as 82.7% was prepared and printed on MgTiO3 ceramics. The thick silver film exhibited a low resistivity of 1.81 μΩ·cm and a good adhesive strength of 39.4 N mm−2, demonstrating broad development prospects in electronic packaging and filters.
Table 4. Comparison between this paper and previous reports on the conductivity and adhesion of conductive silver paste.
| Substrate | Sintering procedure | Resistivity (μΩ·cm) | Sheet Resistance (mΩ/□) | Adhesion (Mpa = N/mm2) |
|---|---|---|---|---|
| Si [34] | 800 °C/10 min | 2.00 | — | — |
| AlN [35] | 850 °C/10 min | — | 4.00 | 11.7 |
| Si [36] | 800 °C/10 min | — | 5.90 | — |
| AlN [37] | 850 °C/15 min | — | 5.30 | 12.7 |
| AlN [33] | 850 °C/10 min | — | 0.93 | 6.7 |
| Al2O3 [28] | 600 °C/15 min | 2.50 | — | 28.5 |
| Al2O3 [38] | 850 °C/10 min | 2.87 | — | 14.6 |
| AlN [16] | 850 °C/15 min | — | 0.90 | 15.3 |
| MgTiO3 | 830 °C/10 min | 1.81 | 2.74 | 39.4this work |
3.3. Influence of sintering temperature on conductivity and adhesion of the silver film
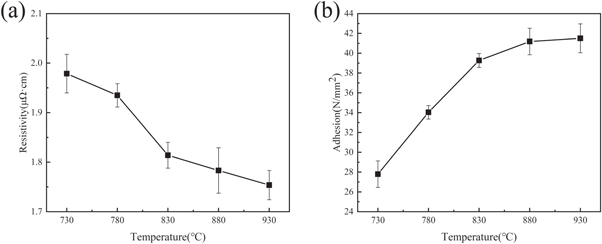
Figure 7 shows the change in the resistivity and adhesion of the silver films after sintering of silver pastes with a glass powder content of 1.1 wt% at 730 °C, 780 °C, 830 °C, 880 °C, or 930 °C. As the sintering temperature increased from 730 °C to 930 °C, the resistivity decreased from 1.97 μΩ·cm to 1.76 μΩ·cm. To analyze the reason for the variation in the resistivity of silver films with temperature, the surface morphologies of silver films obtained under different sintering conditions were studied. As shown in figure 8, the silver film became denser with the increase in temperature, resulting in a decrease in resistivity [39]. At a sintering temperature of 730 °C, fewer sintering necks between silver particles and smaller grain sizes were observed because, under this sintering condition, the softening degree of glass was low, the fluidity was poor, the amount of liquid phase decreased, the silver powder could not be completely wetted, and the high-quality silver network could not be formed. With the increase in sintering temperature, the glass can thoroughly wet the silver powder, drive the rearrangement of the silver powder, and form a denser thick silver film. This phenomenon is a reasonable explanation for the change in resistivity observed in figure 7(a).
Figure 7. (a) Resistivity and (b) adhesion of silver films formed at different sintering temperatures of silver paste with glass powder content of 1.1 wt%.
Download figure:
Standard image High-resolution imageFigure 8. SEM images of the sintered surface of the silver film with a glass content of 1.1 wt% at different temperatures. (a) 730 °C, (b) 780 °C, (c) 830 °C, (d) 880 °C, (e) 930 °C.
Download figure:
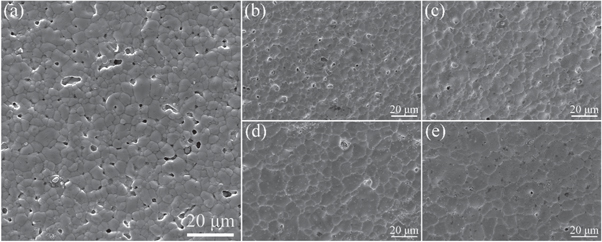
Standard image High-resolution imageAs shown in figure 7(b), the adhesion was significantly improved when the sintering temperature increased from 730 °C to 930 °C. The adhesive strengths of samples sintered at 730 °C, 780 °C, 830 °C, 880 °C, and 930 °C were 27.9 N mm−2, 34 N mm−2, 39.4 N mm−2, 41.2 N mm−2, and 41.3 N mm−2, respectively. The adhesion of the silver film was the weakest at a sintering temperature of 730 °C. The cross-section microstructure of the material is shown in figure 9, in which obvious cracks are visible at the interface between the silver layer and MgTiO3 ceramic substrate. When the sintering temperature was low, the viscosity of the glass was high, and the fluidity was poor. Then, only a small amount of glass could flow to the base connecting the metal layer and the substrate, resulting in poor adhesion of the silver film. At sintering temperatures of 780 °C and 830 °C, the adhesion of the silver layer was improved, and the cracks between the silver layer and the substrate were reduced. Because the glass fluidity gradually improved with the sintering temperature, the amount of glass powder flowing to the MgTiO3 ceramic substrate increased, which resulted in a larger contact area between the silver film and the glass interface layer. With the sintering temperature increased to 880 °C and 930 °C, the adhesive strength of the silver film further increased to 41.2 N mm−2 and 41.3 N mm−2, respectively. However, the improvement was only marginal. The adhesion of the silver film increased with the increase in temperature, which may also be attributed to the change in the density of the silver film [16], as shown in figure 8. As the temperature increases, the glass melts into a liquid phase, which provides good wettability to the MgTiO3 ceramic substrate, resulting in a firmer bond between the silver film and the substrate.
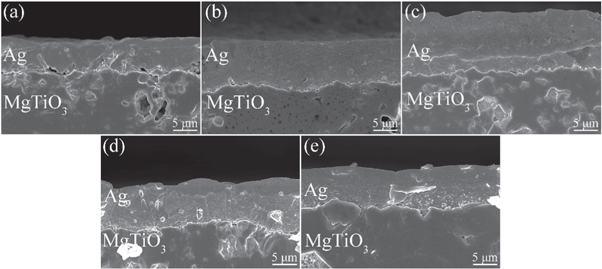
Figure 9. SEM images of the interface between the silver film and MgTiO3 substrate (glass content is 1.1wt%). (a) 730 °C, (b) 780 °C, (c) 830 °C, (d) 880 °C, (e) 930 °C.
Download figure:
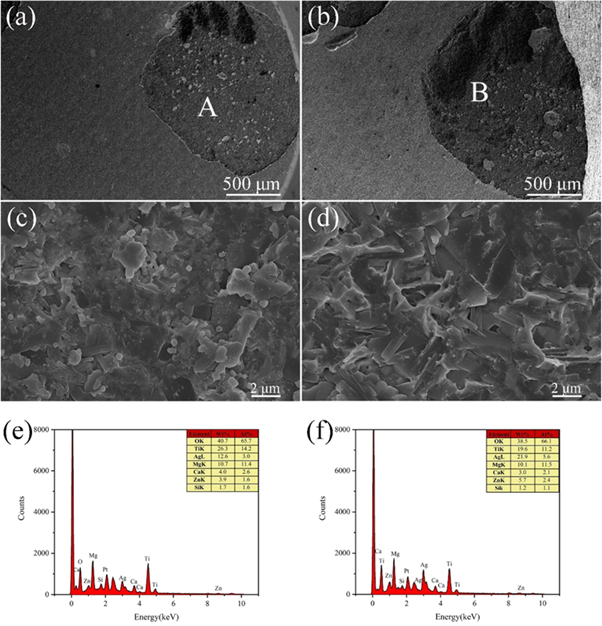
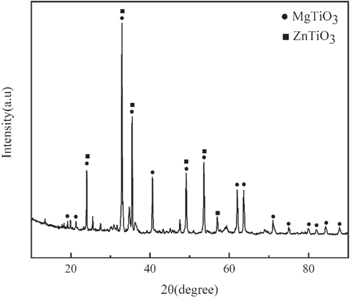
Standard image High-resolution imageFigures 10(a) and (b) show the SEM images of the adhesion test of the silver pastes with glass contents of 0.7 wt% and 1.1 wt% sintered at 830 °C for 10 min EDS chemical element analysis and microscopic analysis were performed on the position of the silver layer after it was pulled off. As shown in figures 10(e) and (f), EDS at the indicated location mainly contained O, Mg, Ti, Zn, Si, Ca, and Ag elements, while the presence of Pt originated from the spraying of Pt during the test. This indicates that the melting of glass powder during the sintering process moistens the silver particles and flows to the substrate to also moisten the substrate and provide better adhesion. In addition to the physical bonding between the glass material and the MgTiO3 ceramic substrate through capillary action, the chemical reaction between ZnO in the glass powder and the MgTiO3 ceramic substrate to form ZnTiO3 might also contribute to the enhanced adhesion. To verify the formation of ZnTiO3, glass powder was directly coated on MgTiO3 ceramics and sintered at 830 °C for 10 min. The XRD results of the obtained material, as shown in figure 11, confirmed the formation of ZnTiO3. Due to the generation of ZnTiO3, the interface between the glass and the MgTiO3 ceramic substrate was strengthened. Fractures mainly occurred at the interface between the silver layer and the glass rather than at the interface between the glass and the ceramic substrate [28]. Therefore, the ZnO-B2O3-SiO2 glass in high-temperature sintered silver paste significantly improves the bonding force between silver paste and MgTiO3 ceramic substrate because the second phase of ZnTiO3 is formed at the interface between silver and the MgTiO3 ceramic, which dramatically improves the bonding strength of the silver film.
Figure 10. (a) Scanning electron microscopy (SEM) of the drawing point of silver film with a glass content of 0.7 wt%, (c) the enlarged SEM image of point A in (a), (e) the EDS of (c); (b) An SEM image of the drawing point of silver film with a glass content of 1.1 wt%, (d) an enlarged SEM image of point B in (b), and (f) an EDS image of (d).
Download figure:
Standard image High-resolution imageFigure 11. XRD pattern of glass powder sintered with MgTiO3 ceramic at 830 °C for 10 min.
Download figure:
Standard image High-resolution image4. Conclusion
In this study, ZnO-B2O3-SiO2 glass with a transition temperature (Tg) of 579 °C and a stability (ΔT) of 105 °C was prepared by high-temperature melting and cold extraction as an inorganic bonding phase for high-temperature conductive silver paste. The resistivity of the silver film decreased from 1.97 μΩ·cm to 1.76 μΩ·cm, and the adhesive strength increased from 27.9 N mm−2 to 41.3 N mm−2 as the sintering temperature increased from 730 °C to 930 °C. When silver paste with a glass content of 1.1 wt% was sintered at 830 °C for 10 min, the silver film exhibited a low resistivity of 1.81 μΩ·cm (1.65 μΩ·cm for silver) and a good adhesive strength of 39.4 N mm−2. During the sintering process, the glass melts and wets the silver powder, which promotes the sintering of the silver powder to form a dense network structure and improves the electrical conductivity of the silver film. In addition, the glass flows toward the MgTiO3 ceramic substrate, and the chemical reaction between the glass and the substrate results in the formation of ZnTiO3 at the interface, which greatly improves the bonding strength of the silver film. Therefore, the glass powder greatly influence the conductivity of thick silver film and the adhesion between the thick silver film and the MgTiO3 ceramic substrate.
Acknowledgments
This work was financially supported by the Technology Projects of Yunnan Province (Grant No. 202101BC070001-017 and Grant No. 202102AB080008). The Fundamental Research Project of Yunnan Province‐General Project under Grant No. 202001AT070061 and the Demonstration construction plan of Kunming Science and Technology Innovation Center under Grant No. 2019‐1‐G‐25318000003420.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.