The Effects of Solvent on Superhydrophobic Polyurethane Coating Incorporated with Hydrophilic SiO2 Nanoparticles as Antifouling Paint
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
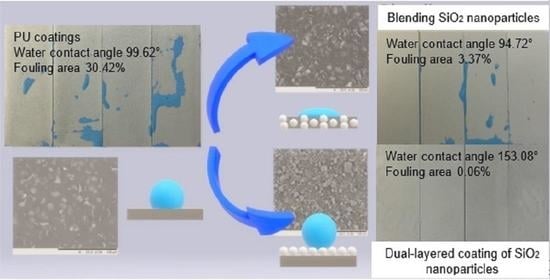
2.2. Blending-Spray Coating Methodology
2.3. Dual-Layer Spray Coating Methodology
2.4. Coating Characterization
2.5. Fouling Test
3. Results and Discussion
3.1. Characteristics of PU Coatings Incorporated with Silica Nanoparticles
3.2. Antifouling Properties of PU Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cassie, A.B.D.; Baxter, S. Wettability of porous surface. Trans. Faraday Soc. 1944, 40, 546–550. [Google Scholar] [CrossRef]
- Karmouch, R.; Ross, G.G. Superhydrophobic wind turbine blade surfaces obtained by a simple deposition of silica nanoparticles embedded in epoxy. Appl. Surf. Sci. 2010, 257, 665–669. [Google Scholar] [CrossRef]
- De Francisco, R.; Tiemblo, P.; Hoyos, M.; González-Arellano, C.; García, N.; Berglund, L.; Synytska, A. Multipurpose ultra and superhydrophobic surfaces based on oligodimethylsiloxane-modified nanosilica. ACS Appl. Mater. Interfaces 2014, 6, 18998–19010. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, W.; Wang, L.; Wang, J.; Wang, S.; Liu, G. A mechanically and chemically stable superhyropobic coating for preventing marine atmospheric corrosion. Surf. Interfaces 2021, 27, 101537. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Repel. Mater. 2015, 347, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Husni, H.; Nazari, M.; Yee, H.; Rohim, R.; Yusuff, A.; Ariff, M.A.M.; Ahmad, N.; Leo, C.; Junaidi, M. Superhydrophobic rice husk ash coating on concrete. Constr. Build. Mater. 2017, 144, 385–391. [Google Scholar] [CrossRef]
- López-Ortega, A.; Areitioaurtena, O.; Alves, S.; Goitandia, A.; Elexpe, I.; Arana, J.; Bayón, R. Development of a superhydrophobic and bactericide organic topcoat to be applied on thermally sprayed aluminum coatings in offshore submerged components. Prog. Org. Coat. 2019, 137, 105376. [Google Scholar] [CrossRef]
- Chen, H.; Wang, F.; Fan, H.; Hong, R.; Li, W. Construction of MOF-based superhydrophobic composite coating with excellent abrasion resistance and durability for self-cleaning, corrosion resistance, anti-icing, and loading-increasing research. Chem. Eng. J. 2021, 408, 127343. [Google Scholar] [CrossRef]
- Penna, M.O.; Silva, A.A.; Rosário, F.F.D.; Camargo, S.D.S.; Soares, B.G. Organophilic nano-alumina for superhydrophobic epoxy coatings. Mater. Chem. Phys. 2020, 255, 123543. [Google Scholar] [CrossRef]
- Cao, C.; Yi, B.; Zhang, J.; Hou, C.; Wang, Z.; Lu, G.; Huang, X.; Yao, X. Sprayable superhydrophobic coating with high processibility and rapid damage-healing nature. Chem. Eng. J. 2020, 392, 124834. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; Gao, A.; Zhang, Q.; Cui, J.; Zhao, S.; Zhan, X.; Yan, Y. Bio-inspired underwater superoleophobic PVDF membranes for highly-efficient simultaneous removal of insoluble emulsified oils and soluble anionic dyes. Chem. Eng. J. 2019, 369, 576–587. [Google Scholar] [CrossRef]
- West, J.; Critchlow, G.; Lake, D.; Banks, R. Development of a superhydrophobic polyurethane-based coating from a two-step plasma-fluoroalkyl silane treatment. Int. J. Adhes. Adhes. 2016, 68, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Carreño, F.; Gude, M.R.; Calvo, S.; De La Fuente, O.R.; Carmona, N. Synthesis and characterization of superhydrophobic surfaces prepared from silica and alumina nanoparticles on a polyurethane polymer matrix. Prog. Org. Coat. 2019, 135, 205–212. [Google Scholar] [CrossRef]
- Najafpour, S.; Moosavi, A.; Najafkhani, H. Condensation enhancement on hydrophobic surfaces using electrophoretic method and hybrid paint coating. Heat Transf. Eng. 2021, 48, 1557–1572. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, Y.; Wang, Q.; Wang, T. Superhydrophobic polyurethane and silica nanoparticles coating with high transparency and fluorescence. J. Appl. Polym. Sci. 2013, 129, 2959–2965. [Google Scholar] [CrossRef]
- Ren, T.; He, J. Substrate-versatile approach to robust antireflective and superhydrophobic coatings with excellent self-cleaning property in varied environments. ACS Appl. Mater. Interfaces 2017, 9, 34367–34376. [Google Scholar] [CrossRef]
- Yousefi, E.; Ghadimi, M.R.; Amirpoor, S.; Dolati, A. Preparation of new superhydrophobic and highly oleophobic polyurethane coating with enhanced mechanical durability. Appl. Surf. Sci. 2018, 454, 201–209. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Y.; Gao, B.; Wan, Y.; Li, Y.C.; Xu, J.; Zhao, Q. Biomimetic superhydrophobic biobased polyurethane-coated fertilizer with atmosphere “outerwear”. ACS Appl. Mater. Interfaces 2017, 9, 15868–15879. [Google Scholar] [CrossRef]
- Zhao, B.; Jia, R. Preparation of superhydrophobic films based on waterborne polyurethane and their hydrophobicity characteristics. Prog. Org. Coat. 2019, 135, 440–448. [Google Scholar] [CrossRef]
- Hamzah, N.; Leo, C.P. Membrane distillation of saline with phenolic compound using superhydrophobic PVDF membrane incorporated with TiO2 nanoparticles: Separation, fouling and self-cleaning evaluation. Desalination 2017, 418, 79–88. [Google Scholar] [CrossRef]
- Hamzah, N.; Leo, C.P.; Ooi, B.S. Superhydrophobic PVDF/TiO2 -SiO2 Membrane with hierarchical roughness in membrane distillation for water recovery from phenolic rich solution containing surfactant. Chin. J. Polym. Sci. 2019, 37, 609–616. [Google Scholar] [CrossRef]
- Hamzah, N.; Nagarajah, M.; Leo, C.P. Membrane distillation of saline and oily water using nearly superhydrophobic PVDF membrane incorporated with SiO2 nanoparticles. Water Sci. Technol. 2018, 78, 2532–2541. [Google Scholar] [CrossRef]
- Tan, H.F.; Tan, W.L.; Hamzah, N.; Ng, M.H.K.; Ooi, B.S.; Leo, C.P. Membrane distillation crystallization using PVDF membrane incorporated with TiO2 nanoparticles and nanocellulose. Water Sci. Technol. Water Supply 2020, 20, 1629–1642. [Google Scholar] [CrossRef] [Green Version]
- Janardhan, R.; Ramamurthy, K.; Anand, J. Solution properties of polyurethane. Polym. Test. 1994, 13, 397–404. [Google Scholar] [CrossRef]
- Monaghan, S.; Pethrick, R.A. Solvent effects on cure in a 2K polyurethane-mechanical and dielectric studies. Ind. Eng. Chem. Res. 2012, 51, 11038–11044. [Google Scholar] [CrossRef]
- Jiang, R.; Hao, L.; Song, L.; Tian, L.; Fan, Y.; Zhao, J.; Liu, C.; Ming, W.; Ren, L. Lotus-leaf-inspired hierarchical structured surface with non-fouling and mechanical bactericidal performances. Chem. Eng. J. 2020, 398, 125609. [Google Scholar] [CrossRef]
- Li, L.; Li, B.; Dong, J.; Zhang, J. Roles of silanes and silicones in forming superhydrophobic and superoleophobic materials. J. Mater. Chem. A 2016, 4, 13677–13725. [Google Scholar] [CrossRef]
- Zheng, H.; Pan, M.; Wen, J.; Yuan, J.; Zhu, L.; Yu, H. Robust, Transparent, and superhydrophobic coating fabricated with waterborne polyurethane and inorganic nanoparticle composites. Ind. Eng. Chem. Res. 2019, 58, 8050–8060. [Google Scholar] [CrossRef]






| Sample | Silica Loading (wt.%) | WCA (°) |
|---|---|---|
| R-0 | 0 | 99.62 ± 3.38 |
| N/5.0 | 5.0 | 98.54 ± 10.85 |
| N/7.0 | 7.0 | 94.72 ± 6.00 |
| S/5.0 | 5.0 | 104.03 ± 14.40 |
| S/7.0 | 7.0 | 110.17 ± 4.85 |
| Sample | Silica Loading (wt.%) | Solvent | WCA (°) |
|---|---|---|---|
| E/20/0.1 | 0.1 | Ethanol | 69.81 ± 10.68 |
| E/20/0.5 | 0.5 | Ethanol | 53.94 ± 6.34 |
| E/20/1.0 | 1.0 | Ethanol | 37.74 ± 19.92 |
| E/20/2.0 | 2.0 | Ethanol | 18.04 ± 1.83 |
| T/20/0.1 | 0.1 | THF | 100.69 ± 3.03 |
| T/20/0.5 | 0.5 | THF | 123.86 ± 13.12 |
| T/20/1.0 | 1.0 | THF | 143.95 ± 10.19 |
| T/20/2.0 | 2.0 | THF | 148.81 ± 2.96 |
| P/20/0.1 | 0.1 | Thinner | 85.87 ± 5.41 |
| P/20/0.5 | 0.5 | Thinner | 142.00 ± 2.79 |
| P/20/1.0 | 1.0 | Thinner | 153.08 ± 0.51 |
| P/20/2.0 | 2.0 | Thinner | 152.71 ± 1.81 |
| Samples | Method | Chemicals | Average WCA (°) |
|---|---|---|---|
| R-0 | Spray coating | - | 99.62 ± 3.38 |
| N/7.0 | Blending-spray coating | - | 94.72 ± 6.00 |
| S/7.0 | Blending-spray-silane post-treatment | perfluorooctyltriethoxy silane | 110.17 ± 4.85 |
| E/20/2.0 | Dual-layer spray coating | ethanol | 18.04 ± 1.83 |
| T/20/2.0 | tetrahydrofuran | 148.81 ± 2.96 | |
| P/20/2.0 | paint thinner | 152.71 ± 1.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanthasamy, R.; Algarni, M.; Peng, L.C.; Zakaria, N.A.; Zwawi, M. The Effects of Solvent on Superhydrophobic Polyurethane Coating Incorporated with Hydrophilic SiO2 Nanoparticles as Antifouling Paint. Polymers 2023, 15, 1328. https://doi.org/10.3390/polym15061328
Kanthasamy R, Algarni M, Peng LC, Zakaria NA, Zwawi M. The Effects of Solvent on Superhydrophobic Polyurethane Coating Incorporated with Hydrophilic SiO2 Nanoparticles as Antifouling Paint. Polymers. 2023; 15(6):1328. https://doi.org/10.3390/polym15061328
Chicago/Turabian StyleKanthasamy, Ramesh, Mohammed Algarni, Leo Choe Peng, Nur Ain Zakaria, and Mohammed Zwawi. 2023. "The Effects of Solvent on Superhydrophobic Polyurethane Coating Incorporated with Hydrophilic SiO2 Nanoparticles as Antifouling Paint" Polymers 15, no. 6: 1328. https://doi.org/10.3390/polym15061328