Abstract
The search for higher energy density, safer, and longer cycling-life energy storage systems is progressing quickly. One-dimensional (1D) nanomaterials have a large length-to-diameter ratio, resulting in their unique electrical, mechanical, magnetic and chemical properties, and have wide applications as electrode materials in different systems. This article reviews the latest hot topics in applying 1D nanomaterials, covering both their synthesis and their applications. 1D nanomaterials can be grouped into the categories: carbon, silicon, metal oxides, and conducting polymers, and we structure our discussion accordingly. Then, we survey the unique properties and application of 1D nanomaterials in batteries and supercapacitors, and provide comments on the progress and advantages of those systems, paving the way for a better understanding of employing 1D nanomaterials for energy storage.
Export citation and abstract BibTeX RIS
1. Introduction
With the ever-increasing demand for green energy, driven by the rapid growth of the global economy and population, clean and renewable energy storage is considered one of the most essential concerns for today's energy-conscious society [1–3]. Among the various kinds of energy sources, electricity is typically considered the most convenient, because it can be easily converted into other forms of energy. This has given rise to enduring research into and industrial fervor for electric energy storage technologies, specifically rechargeable batteries and supercapacitors, which play a crucial role in both stationary and mobile applications [4, 5]. Since Sony succeeded in the first commercialization of rechargeable Li-ion batteries (LIBs) in 1991, we have witnessed earth-shaking changes in our daily lives [6–8]. LIBs are well received by our modern society, as they not only power cell phones and laptops, but they are finding a place in the hybrid and electric vehicle market [8–11]. LIBs are doing a great job in beginning the replacement of the current oil-driven vehicles in order to reduce the consequent generation of CO2 [12, 13]. In order to meet future requirements for portable electronics, transportation, and large-scale energy storage systems [1–4, 14, 15], ideal batteries should have even higher energy density, longer cycle stability, wider environmental compatibility, broader worldwide consumer distribution, higher safety, and lower production costs [16]. In brief, extreme improvements in the performance of electrode materials and the design of electrode architectures are highly desirable.
Nanostructures, i.e. intriguing intermediates between microscopic and molecular structures, have attracted considerable interest due to their unique and fascinating properties, as well as their extensive applications with superior performance to their bulk counterparts [17]. These unique structures have huge potential in advancing a brand-new generation of energy storage devices whose capacities approach theoretical values. 1D nanomaterials, including nanowires (NWs) [18], nanofibers [19], nanorods, nanobelts, and nanotubes (NTs) [20], excel in terms of larger surface areas, extra surface-active sites, and better permeability, all of which can incomparably increase the energy density, power density, and cycling performance for energy storage [2, 21]. Therefore, nanomaterials have drawn great attention for their far-reaching scope in potential applications in rechargeable batteries and supercapacitors. The various synthesis strategies can be grouped into the following major categories: liquid-phase methods [22–25], electrospinning methods [26–28], template-assisted methods [29, 30], chemical etching methods, and chemical deposition methods [31, 32]. We list typical examples in tables 1 and 2 to briefly describe the synthesis strategies, material morphology, and application of 1D nanomaterials for LIBs and supercapacitors.
Table 1. Electrode materials, morphologies, synthesis strategies, and electrochemical performance of 1D nanomaterials in LIBs (E = electrospinning method, T = template-assisted method, CE = chemical etching method, L = liquid-phase method, and CD = chemical deposition method).
| Electrode material | Morphology | Synthesis strategy | Reversible capacity/mA h−1 g−1 | Specific current/mA g−1 | Ref. |
|---|---|---|---|---|---|
| C | MWNTs | T | 400 | 30 | [37] |
| C | SWNTs | CE | 700 | 50 | [38] |
| N-doped C | NWs | E | 632 | 1000 | [116] |
| Si | NWs | CD | 3100 | 2100 | [51] |
| Si | NWs | T and CE | 3247 | 600 | [55] |
| Si | nanorods | T | 2411 | 410 | [59] |
| Si | NTs | T | 2700 | 210 | [100] |
| Si/SiOx | NWs | CE | 1503 | 600 | [64] |
| Sn-doped TiO2 | NTs | L | 252 | 50 | [117] |
| Co3O4@α-Fe2O3 | NWs | L | 980 | 100 | [18] |
| VO2(B) | NWs@NBs | L | 117 | 1000 | [70] |
| MnO2/PEDOT | NWs | T | 300 | 3000 | [85] |
| α-Fe2O3/SnO2 | nanorods | CD | 820 | 1000 | [77] |
Table 2. Electrode materials, morphologies, synthesis strategies, and electrochemical performance of 1D nanomaterials in supercapacitors. (The corresponding synthesis methods are the same as in table 1.)
| Electrode material | Morphology | Synthesis strategy | Capacity | Electrolyte | Ref. |
|---|---|---|---|---|---|
| C | VA-NTs | CD | 365 F g−1 at 210 mA g−1 | 1 M H2SO4 | [47] |
| N-doped C | NTs | T | 210 F g−1 at 500 mA g−1 | 6 M KOH | [108] |
| Co3O4 | NWs | L | 754 F g−1 at 2 A g−1 | 2 M KOH | [67] |
| NiCo2O4 | NTs | E | 1756 F g−1 at 1 A g−1 | 2 M KOH | [69] |
| ZnO/Mn3O4 | NTs | T | 441.4 F g−1 at 2 mV s−1 | 0.5 M Na2SO4 | [71] |
| C/NiO | NTs | CD | 1657 F g−1 at 500 mA g−1 | 6 M KOH | [32] |
| CoO@PPy | NWs | CD | 2223 F g−1 at 1 mA g−1 | 3 M NaOH | [80] |
| MnO2/PEDOT | NWs | T | 210 F g−1 at 1 mA cm−2 | 1 M Na2SO4 | [115] |
| PANI/C | nanofibers | L | 480 F g−1 at 100 mA g−1 | 3 M NaOH | [87] |
| PANI/C | hybrid structure | L | 448 F g−1 at 1 A g−1 | 0.5 M H2SO4 | [25] |
| PANI | NWs | CD | 950 F g−1 at 1 A g−1 | 1 M HClO4 | [100] |
A handful of magnificent reviews are available which summarize the synthesis and applications of 1D nanomaterials, however, these tend to focus either on a specific category of materials or on a certain application. Herein, we hope to provide a thorough and up-to-date inspection of the diverse categories and applications of 1D nanomaterials. Considering the rapid development of nanotechnology, we believe that this review could serve as both a scientific introduction for newcomers to the relevant fields and a comprehensive reference for experienced researchers.
This article conducts a broad overview of the latest development of 1D nanomaterials in the field of energy storage. First, we provide a brief summary of the structural features of 1D nanostructures and their advantages for electrochemical energy storage. Second, the recent progress of 1D nanomaterials will be extensively discussed in accordance with the categories carbon, silicon, metal oxides, and conducting polymers. Then we deliberate their corresponding applications in electrochemical energy storage as rechargeable batteries and supercapacitors. In addition, we draw up a table to summarize the details of important electrode materials, morphologies, synthesis strategies, and electrochemical performance. Finally, this review concludes with a concise perspective on the prospects for 1D nanomaterials.
2. Electrodes from 1D nanomaterials
2.1. Carbon
Carbon NTs (CNTs) are a class of tubular carbon materials with a graphitic structure [21]. CNTs have been proposed as electrodes for energy storage [33]. At the molecular level, CNTs are usually folded up from one or more sheets of graphene. CNTs can be divided into single-wall (SWNT) or multi-wall (MWNT) NT structures. CNTs are 1D nanostructures with a significant length-to-diameter ratio, in which the carbon atoms are arranged as a hexagonal lattice, with each carbon atom surrounded by three nearest-neighbor atoms, resulting from special covalent sp2 carbon bonding [34, 35]. In addition, CNTs possess unique size-/surface-dependent properties which are useful for efficient energy storage. Some specific features result from their excellent electrical properties as well.
LIBs have become the common power source for portable applications, such as electronics and electric vehicles [16]. Increasing the specific capacity of the battery electrodes and shortening the charging time are both essential aims for electric vehicles. In addition, higher energy densities, a longer cycle life, and lower costs are also important for electrochemical energy storage [1]. Given that CNTs have unique properties of high electrical conductivity, great charge transport capability, a large specific surface area, high mesoporosity, and high electrolyte accessibility, they have been considered attractive materials for LIBs. In recent years, CNTs have been used as host materials and conductive additives [36]. Herein, we present some research on LIBs. It is reported that a lot of Li+ can be stored in the central core, the interlayer space (for MWNTs), or the empty space between the NTs when they are assembled in bundles. However, reversible capacities of only 400 mAh g−1 for MWNTs and 500 mAh g−1 for SWNTs in LIBs have been reported [37]. It has been assumed that the capacity can be further increased by opening or cutting the CNTs. In order to obtain a reversible capacity of about 700 mAh g−1 for SWNTs, Shimoda et al [38] used strong acids to open the tubes and reduced their length. Moreover, Gao et al [39] achieved a reversible capacity of 1000 mAh g−1 by ball milling SWNTs. At the same time, a capacity retention of about 60% was reported upon charge/discharge current density increases from 50 mA g−1 to 500 mA g−1, demonstrating that CNT electrodes showed excellent rate performance.
It is well known, however, that carbon-based materials suffer from irreversible capacity upon charge and discharge. The irreversible capacity is mainly derived from the formation of a passivating layer, which is called the solid electrolyte interphase (SEI). In addition, the kinetics of the Li+ intercalation/de-intercalation reactions result in undesired voltage hysteresis. Some researchers investigated the effect of the CNTs' orientation, proposing the use of vertically aligned CNTs (VA-CNTs). Since then, VA-CNTs have received more and more attention in the field of energy storage, in particular to serve as a novel anode electrode material for LIBs [40].
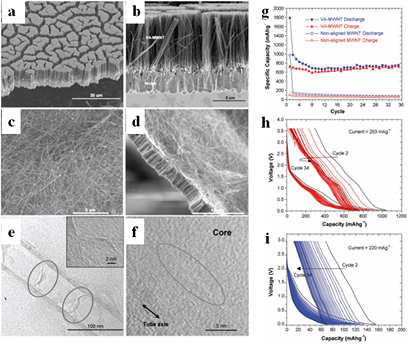
Welna et al [41] reported that, at low specific currents, VA-MWNTs show highly reversible specific capacities (i.e. up to 782 mAh g−1 at 57 mA g−1). Interestingly, at very high discharge rates, the VA-MWNT electrodes retained a moderate specific capacity due to their aligned nature (166 mAh g−1 at 26 A g−1). The VA-MWNTs were supported at their base by a thin nickel metal film, which helped to maintain their alignment (figures 1(a)–(f)). Owing to the excellent contact with the VA-MWNTs, the nickel film acted as a suitable current collector. The results showed that the alignment of the MWNTs in the direction of the ion flow in the battery cell significantly increased the accessibility of the electrode interior to the electrolyte. TEM images (figures 1(e) and (f)) show that the internal core of the NTs contains irregular structural features such as loose graphene layers (red circles) and graphene NT edges (blue circle). These structural features significantly increase the effective surface area of the NTs and provide more defect sites at which lithium ions may be adsorbed to the surfaces of loosely bound graphene layers or to hydrogen-containing edge carbon sites. Additionally, the intimate contact between each of the vertically aligned NTs and the nickel metal current collector provides a low resistance pathway, allowing for effective connectivity throughout the electrode. The Li-ion storage capacity and rate capability of the aligned NTs were found to be significantly improved compared to their non-aligned counterparts. The specific capacities of both the VA-MWNTs and the non-aligned MWNTs as a function of cycle number are reported in figure 1(g), while the voltage profiles for these cycles are shown in figures 1(h) and (i). After the first cycle, the reversible capacity of the VA-MWNTs decreases from 980 mAh g−1 to a minimum near the tenth cycle, after which it increases slightly and stabilizes near 750 mAh g−1. The reversible capacity for the non-aligned MWNTs continuously decreases from 158 mAh g−1 to 58 mAh g−1 after 34 cycles [41]. Owing to their large specific capacity, VA-CNTs with high specific capacity are being widely researched as potential LIB electrode materials, although there remain some concerns about their volumetric capacity and first cycle reversibility, the latter requiring pre-lithiation approaches.
Figure 1. (a) and (b) SEM images of VA-MWNTs, and (c) and (d) non-aligned MWNTs. (e) TEM images of VA-MWNTs, showing the graphene NT edges (f) within the internal NTs structure. (g) Specific capacities and voltage profiles of cycles 2–34 for (h) VA-MWNTs and (i) non-aligned MWNT electrodes. Reprinted from [41], Copyright 2011, with permission from Elsevier.
Download figure:
Standard image High-resolution imageSupercapacitors offer, with respect to batteries, higher power capability [1, 42]. As mentioned earlier, the advantages of CNTs include their high electrical conductivity, high charge transport capability, high mesoporosity, and excellent electrolyte accessibility, all contributing to high-power performance [43]. Thus, CNTs have been used to fabricate supercapacitors. Lee et al [44] fabricated a supercapacitor using SWNT electrodes and a KOH electrolyte. Their report showed a promising power density (20 kW kg−1) with a maximum energy density of about 10 Wh kg−1. The equivalent series resistance of the supercapacitor was very small. It has also been shown that composites of large SWNTs, i.e. with diameters of 10–20 nm, and poly(vinylidene chloride) carbonized at 1000 °C could offer a specific capacitance of 180 F g−1, corresponding to 6.5 Wh kg−1, at a power density of 20 kW kg−1.
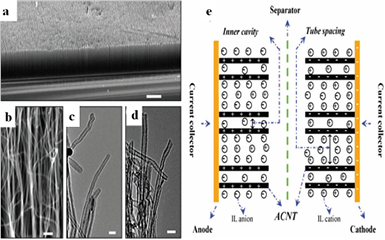
Randomly oriented CNTs show, however, the entangled morphology affecting the performance of the supercapacitor. VA-CNTs have also been extensively studied in recent years [36, 45], because they are better-structured materials for supercapacitors. For example, Dai et al [43] investigated VA-CNTs with well-defined tube spacing (figure 2). These structures provide easier electrolyte accessibility and, in addition, better charge storage/delivery properties. Owing to the special structures of CNTs, the ions can not only be delivered rapidly through each tube in the electrode, but also offering excellent power density for supercapacitors [43, 46]. Hence, various researchers have confirmed the improved rate capability of VA-CNTs. Chen et al [47] obtained a VA-CNT array electrode, offering 365 F g−1, prepared via template-assisted chemical vapor deposition (CVD) in 1 M H2SO4.
Figure 2. (a) and (b) SEM images of a plasma-etched, VA-CNT electrode at different magnifications (scale bars: 100 µm and 100 nm, respectively). TEM images of the CNTs before (c) and after (d) plasma etching (scale bar: 20 nm). The MWCNT array is highly aligned with the tube length of ≈150 µm and the outer and inner diameters of approximately 10 nm and 5 nm, respectively. (e) Schematic representation of a supercapacitor based on plasma-etched VA-MWNT electrodes and ionic liquid electrolyte. Reprinted from [43], Copyright 2009, with permission from Elsevier.
Download figure:
Standard image High-resolution image2.2. Silicon
Currently, graphite is widely used as anode material for LIBs. However, to satisfy the increasing demand in energy storage, silicon is regarded as one of the most promising anode electrode materials, due to its high theoretical capacity of 4200 mAh g−1 (i.e. ten times higher than that of graphite) [48]. Nonetheless, silicon shows a large volume change (400%) upon lithiation, which leads to Si pulverization and capacity loss upon cycling (insertion and extraction of lithium ions) [49]. In addition, Si has poor rate capability due to the low diffusivity of lithium ions. 1D Si nanostructures may offer improved rate capability and cyclability for three reasons: (i) the high surface-to-volume ratio better accommodates the enormous volume change of Si during cycling, which limits mechanical pulverization and improves cyclability; (ii) the large specific surface area is beneficial to rapid Li-ion transfer kinetics at the interface; and (iii) the nanosized radial dimension makes lithium diffusion more efficient due to shortened Li-ion diffusion length.
2.2.1. Silicon nanowires.
The first report of the use of 1D Si nanostructures as anode electrode materials for LIBs appeared in 2007, when Cui et al [50] reported the synthesis of silicon NWs (SiNWs) on stainless steel substrate current collectors. The SiNWs exhibited a very high initial discharge capacity (3124 mAh g−1) and reasonable coulombic efficiency (73%). The constant discharge capacity of over 3000 mAh g−1 was retained from the second to the tenth cycle. Pribat et al [51] presented a Si anode material composed of highly interconnected NWs, which showed around 100% charge capacity retention after 40 cycles at a 0.5 C rate. This electrode material could be cycled at an 8 C rate without damage. Zhi et al [52] reported a SiNW/graphene/reduced graphene oxide (RGO) composite (SiNW@G@RGO) via the encapsulation of SiNWs with dual adaptable layers (overlapped graphene (G) sheaths and RGO overcoats). At 2.1 A g−1 the electrodes delivered high reversible specific capacity (1600 mAh g−1) on the basis of the total electrode weight, and about 80% capacity retention after 100 cycles. In this structure, the overlapping graphene sheets could prevent the direct exposure of encapsulated silicon to the electrolyte, which is beneficial for the structural and interfacial stabilization of SiNWs. The flexible RGO overcoating accommodates the volume change of SiNWs@G nanocables during cycling, enabling the structural integrity of the electrode. Laik et al [53] investigated the effect of the NWs' diameter on the electrochemical properties of SiNWs. Three samples (diameters smaller than 65, 210, and 490 nm) were synthesized using gold catalyst with different film thicknesses. A high capacity of 3500 mAh g−1 was achieved for the smallest diameters at a 0.2 C rate, while 2500, 1500, and 500 mAh g−1 were achieved at 1 C, 2.5 C and 5 C, respectively. A high capacity of 2500 mAh g−1 was obtained after 50 cycles at 1.3 C. Yan et al [54] used porous SiNWs made using cheap metallurgical silicon as a starting material. Importantly, a thin oxide layer (~3 nm) was present on the surface of the porous SiNWs. This SiOx coating was reported to constrain the large volume change of Si, thus enhancing the mechanical integrity of the electrode and its cycle performance. Finally, the Si/SiOx NWs exhibited a reversible capacity of 1503 mAh g−1 after 560 cycles at a current density of 600 mA g−1.
2.2.2. Silicon nanotubes.
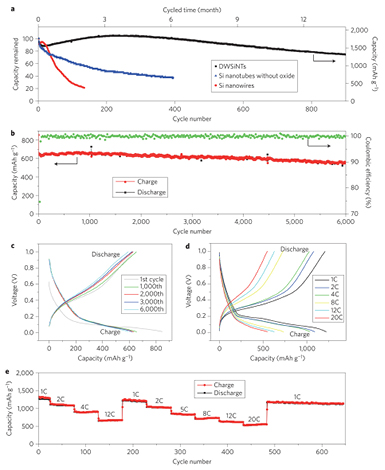
NTs have been a hot topic in the field of 1D Si nanomaterials due to their hollow structure, providing additional space to accommodate the volume change during cycling. Both the inner and outer surface of the tubes are exposed to the electrolyte, which shortens the lithium diffusion length even further. Cho et al [55] synthesized silicon NTs (SiNTs) using a reductive decomposition method and etching. The NTs showed a special capacity of 3247 mAh g−1 and a coulombic efficiency of 89%. At the rate of 5 C, good capacity retention was also obtained. SiNTs show excellent electrical performance, but their low coulombic efficiency is a critical problem that needs to be addressed. In fact, the large interfacial area of the nanotubular geometry leads to extensive degradation of the electrolyte for SEI formation, leading to consumption of lithium ions and, thus, low coulombic efficiency. In addition, the continuous volume change results in cracking of the SEI layer, which is continuously reformed, leading to poor electrochemical performance. An effective method to relieve this inconvenience to some extent consists in using other materials to shield the inner side of the SiNTs. In 2011, Paik et al [56] prepared a novel Si/Ge double-layered NT array (NTA) electrode, which showed better capacity retention and rate performance compared to pure SiNTs. Cui et al [57] reported a double-walled SiNTs (DWSiNTs) anode consisting of silicon on the inner side and silicon oxide on the outer side. The SiOx outer shell layer limits the expansion of the inner silicon materials during insertion of lithium ions, making the SEI layer more stable. DWSiNTs exhibited a high reversible capacity of 1780 mAh g−1 (on the basis of the total weight of Si and SiOx) and an outstanding capacity retention of 79% after 900 cycles at a 0.2 C rate, and the capacity of silicon alone could even achieve as high a value as 2791 mAh g−1. Compared to SiNWs and SiNTs without oxide, the cycling performance of DWSiNTs is obviously improved (figure 3(a)). More importantly, the DWSiNTs showed an excellent capacity retention of 88% even after 6000 cycles at a 10 C rate. Despite the coulombic efficiency of the first cycle being 76% (some lithium ions are consumed by the constraining SiOx layer and initial SEI formation), the average coulombic efficiency of the DWSiNTs electrode is 99.938% from the second to 600th cycles (figure 3(b)). The charge/discharge profile showed no obvious change after 6000 cycles, confirming the stability of the DWSiNT material and resulting in a stable electrochemical performance (figure 3(c)). Between 1 C and 20 C, high and stable capacities between 1200 mAh g−1 and 540 mAh g−1 were accomplished in the DWSiNTs (based on total DWSiNT weight) (figures 3(d) and (e)). Attributed to the thin and stable SEI and the nanoscale thickness of the walls in DWSiNTs, lithium ions can rapidly pass through the SEI layer and constraining SiOx layer to reach the silicon active material, and a remarkable high power rate capability was achieved.
Figure 3. Electrochemical performance of a DWSiNT anode. (a) Capacity retention of different silicon nanomaterials. (b) Charge/discharge capacity and coulombic efficiency of DWSiNTs cycled at 12 C for 6000 cycles. (c) Voltage profiles plotted for the 1st, 1000th, 2000th, 3000th, and 6000th cycles. Galvanostatic charge/discharge profiles (d) and capacity (e) of DWSiNTs cycled at different rates. Reprinted by permission from Macmillan Publishers Ltd: Nature Nanotechnology [57], Copyright 2012.
Download figure:
Standard image High-resolution image2.2.3. Silicon nanorods.
As do the other 1D silicon nanomaterials, nanorods exhibit better electrochemical performance than bulk silicon. Zhou et al [58] prepared mesoporous silicon nanorods through a magnesiothermic reduction process, using CNTs as a template. The resultant nanorods had a high reversible capacity of 1038 mAh g−1 after 170 cycles. Kim et al [59] reported bundle-type silicon nanorods synthesized by etching silicon thick films. This nanostructure improves the coulombic efficiency and cycle performance, and a high capacity of 2411 mAh g−1 was retained at a current density of 410 mA g−1 after 30 cycles.
2.3. Metal oxides
Metal oxides represent a large family of precursors for the development of functional nanomaterials, owing to the fact that oxides are the lowest free-energy states for most metals in the periodic table in the oxidative atmosphere of the Earth. Considering the high specific capacity/capacitance, metal oxides are certainly promising materials for rechargeable batteries and supercapacitors [60, 61]. However, the cycle stability and rate performance of bulk metal oxides cannot meet the requirements of practical applications. 1D nanostructures, which have a larger electrochemically active area, and faster electron transport and ion diffusion, are expected to significantly improve the cycling and rate performance [62]. Therefore, in recent years, 1D metal oxide nanostructures have been intensively investigated [61], such as hierarchical NiCo2O4@MnO2 core/shell heterostructured NW arrays [63], α-Fe2O3/SnO2 nano-heterostructures [64], and hollow Co3O4 NW arrays [65].
Li et al made use of a CNT cathode and TiO2-B NW anode to fabricate a hybrid supercapacitor delivering a specific energy of 12.5 Wh kg−1 at a 10 C rate [66]. Tu et al [67] prepared a free-standing Co3O4 NW array grown on nickel foam via a hydrothermal synthesis method. The Co3O4 NW exhibited superior pseudocapacitive performance with a high capacitance of 754 F g−1 at 2 A g−1 and 610 F g−1 at 40 A g−1 as well as remarkable cycle stability. Wang et al [68] presented a high-efficiency fiber-based electrochemical micro-supercapacitor making use of ZnO NWs as electrodes. The NWs, with diameters ranging from 500 nm to 700 nm and lengths of about 6 µm, had remarkable flexibility. Thus these flexible fiber supercapacitors could be combined with fiber nanogenerators to achieve a wearable power system. However, its capacitance value was relatively low about 0.21 mF cm−2. In order to enhance the specific capacitance of these fiber-based supercapacitors, ZnO NWs grown on plastic wires were coated with MnO2 at room temperature to take advantage of the latter material's pseudocapacitance. This energy storage device displayed a specific capacitance of about 2.0 mF cm−2 at 100 mV s−1, indicating that using an optimized MnO2 coating can further enhance the performance of fiber supercapacitors [68].
Chen and co-workers [69] reported a facile synthesis strategy to fabricate 1D metal composite oxides with complex architectures by using a facile electrospinning technique followed by a thermal treatment process (figures 4(a)–(j)). Adjusting the precursor polymers and heating rate resulted into complex tubular 1D nanostructures ranging from 1D solid NTs, hollow tubes, to tube-in-tubes. By using different polymer compositions, various types of transition metal oxides (TMOs) with 1D nanostructures, including CoMnO4, NiCo2O4, CoFe2O4, NiMn2O4, and ZnMn2O4, have been successfully fabricated. SEM images have clearly shown the morphology of these unique nanostructures. This strategy can be extended to synthesizing a relatively wide range of spinel complex 1D tubular nanostructures, which further demonstrates the generality and feasibility of this method. Owing to their novel and unique structure properties, the tube-in-tube hollow nanostructures exhibited extraordinary electrochemical performance in Li–O2 batteries and asymmetric supercapacitors. In particular, the specific capacitance of the NiCo2O4 tube-in-tube structures reached 1756, 1697, 1610, 1511, and 1459 F g−1 at current densities of 1, 2, 5, 10, and 20 A g−1, respectively. Zheng et al [18] adopted a facile, two-step hydrothermal synthesis of a novel Co3O4/α-Fe2O3 branched NW heterostructure (figures 4(k)–(m)). SEM images have shown uniform Co3O4/α-Fe2O3 branched NW heterostructures. The primary Co3O4 NW trunks could serve as 1D conduction channels for fast Li+ transport, while the secondary Fe2O3 NWs branches provide a high surface area for an enhanced electrolyte interface as well as a large electrochemical capacity for Li+ storage. Furthermore, the space between the α-Fe2O3 branches beneficially reduce the aggregation of primary Co3O4 trunks and alleviate the mechanical stress associated with the volume change induced by Li+ intercalation/extraction, thus leading to improved electrochemical energy storage performance. As a result, the Co3O4/α-Fe2O3 branched NWs showed a high initial discharge capacity (~1534 mAh g−1 at a current density of 100 mA g−1), which stabilized at 980 mAh g−1, i.e. a much higher value than that of pristine Co3O4 NWs (311 mAh g−1) or α-Fe2O3 NWs (75 mAh g−1). The coulombic efficiency was above 95% at each cycle [18]. Moreover, the development of such multi-component, branched NW heterostructures, potentially with additional carbon coating, may allow for more material architectures, leading to new promising material building blocks for electrochemical energy storage.
Figure 4. (a) Schematic of the formation process of TMOs with complex 1D nanostructures. (b) SEM and (c) TEM images of NiCo2O4, (d) SEM and (e) TEM images of CoFeO4, (f) SEM and (g) TEM images of NiMn2O4, and (h) SEM and (i) TEM images of ZnMn2O4. (j) Electrochemical performance of NiCo2O4 tube-in-tube nanostructures at various current densities as supercapitors. Reprinted with permission from [69]. Copyright 2015 American Chemical Society. (k) Schematic of the synthesis of branched Co3O4/α-Fe2O3 NWs: (l) SEM image and (m) cycle performance at current density of 100 mA g−1 as LIB anodes [18]. 2013 © Tsinghua University Press and Springer-Verlag Berlin Heidelberg 2013. With permission of Springer.
Download figure:
Standard image High-resolution imageMai et al [70], using the synergistic effects of different surfactants, designed and constructed a nanoscroll-buffered, hybrid 1D nanostructural VO2(B) (HNS), which was composed of NWs and nanobelts with significant enhancement in electrochemical performance. This novel HNS with a buffered section provides a facile strain relaxation, accommodating the volume variation during charge/discharge, thus improving the structural stability and in turn improving the fast capacity loss and high-rate performance. Moreover, the interior of the nanoscrolls and the interconnected voids between the hybrid nanostructures could shorten the Li-ion diffusion pathway. HNS showed great high-rate performance and long life-cycle performance at a current density of 1000 mA g−1. The initial discharge capacity reached 117 mAh g−1, while after 500 and 1000 cycles the capacity retention was 90% and 82%, respectively. Liu et al [71] synthesized ZnO/Mn3O4 core/shell NTAs via a two-successive-step electrochemical deposition process and heat treatment method. At scan rate of 2 mV s−1 ZnO/Mn3O4 NTAs achieved the specific capacitance of 441.4 F g−1, i.e. far beyond that of ZnO/Mn3O4 nanorod arrays (NRAs) (216.3 F g−1). After 1000 cycles, the specific capacitance of ZnO/Mn3O4 NTAs decreased by only about 7.1%, revealing a long cycle life. These works indicate that the novel 1D architectures offer a very promising design for supercapacitors.
2.4. Conducting polymers
Recently, 1D conducting polymers have received widespread attention as energy conversion and storage materials due to their good mechanical properties, high surface areas, and shortened transport pathways [72]. Wen et al [73] synthesized tubular polypyrrole (T-PPy) and granular polypyrrole (G-PPy). When combined with sulfur, the S/T-PPy composite exhibited a reversible capacity of 500 mAh g−1 after 60 cycles, which was better than that of the S/G-PPy composite. 1D nanostructured T-PPy offers better performance when provided with a well-constructed electric network. Hammond et al [74] assembled electrodes consisting of polyaniline (PANI) nanofibers and multi-wall CNTs (MWCNTs). A high specific capacity (147 mAh g−1) and excellent cycle stability (over 10 000 cycles) were obtained due to the CNTs and redox active PANI nanofibers storing charge through both electrical double layer and Faradaic mechanisms. Lee et al [75] fabricated MnO2 nanoparticle enriched, poly(3,4-ethylenedioxythiophene) (PEDOT) NWs as a cathode material for LIBs by simply soaking the PEDOT NWs in KMnO4 solution. The material offered a high charge capacity (300 mAh g−1) and capacity retention (90%) after 500 cycles.
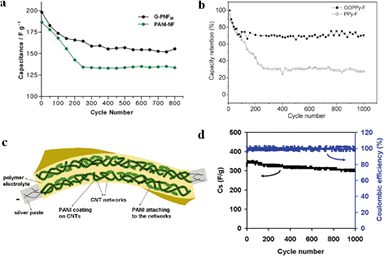
Supercapacitors are another important application of 1D structured conducting polymers. Cho et al [76] reported PEDOT NTs synthesized by an electrochemical template method. The supercapacitor based on PEDOT NTs exhibited high-power performance, maintaining 80% of its maximum energy density at 25 kW kg−1. Wei et al [77] synthesized polypyrrole NW (PPyNW) arrays by electrochemical polymerization strategy. The diameter of the NWs was in the 80–100 nm range, while the length was controlled within the 1–4 µm range. The PPyNW arrays exhibited a high capacitance of 566 F g−1 and retained 70% of their initial capacitance after hundreds of cycles. Compared to pure 1D conducting polymers, the binary or multiple composites based on 1D nanostructured PPy [78–80], PANI [81–84], PEDOT [85, 86], and other materials, are much more competitive for supercapacitor applications. Carbon nanomaterials and TMOs are often used in combination with conducting polymers. Zhang et al [87] presented PANI/graphene nanofiber composites. When used as electrode materials in supercapacitors, high capacitance (480 F g−1) and good cycle stability were obtained at a current density of 0.1 A g−1 (for the best sample). Wu et al [88] synthesized composite films consisting of chemically converted graphene and PANI nanofiber by vacuum filtration. The supercapacitor based on these composite films exhibited high conductivity (5.5 × 102 S m−1) and capacitance (210 F g−1) at a discharge rate of 0.3 A g−1 (figure 5(a)). The remarkable capacitance of 155 F g−1 was maintained after 800 cycles. The performance of 1D conducting polymer nanomaterials was also improved by the introduction of graphene oxide (GO) via a template-free method. The PPy nanofiber/GO nanocomposites eventually showed excellent capacitance, over 500 F g−1. The rate performance and cyclability were also improved because the composites have the unique advantages of both PPy nanofiber and GO (figure 5(b)) [89]. Wei et al [90] synthesized nanocomposites combining 1D conducting PANI NWs with 2D GO nanosheets. The PANI NW arrays aligned vertically on the GO substrate. The hierarchical nanocomposite exhibited enhanced electrochemical capacitance and stability when used as supercapacitor electrode materials. Liu et al [91] reported a novel kind of supercapacitor, which used CNT/PANI nanocomposite thin films as electrodes. The thickness of these electrodes is comparable to that of a piece of standard commercial A4 print paper. These electrodes exhibited remarkable capacitance (350 F g−1), good cycle stability after 1000 cycles, and very small self-discharge (figures 5(c) and (d)). In 2013, Liu et al [80] developed CoO@PPy hybrid NW arrays on nickel foam, exhibiting excellent pseudocapacitive performance, i.e. a remarkable specific capacitance of 2223 F g−1, superior cycle stability (capacitance retention of 99.8% after 2000 cycles), and enhanced rate capability. When used as positive electrodes in aqueous, asymmetric supercapacitors, high energy density (~43.5 Wh kg−1), high power density (~5500 W kg−1 at 11.8 Wh kg−1), and outstanding cyclability (~20 000 times) were achieved.
Figure 5. (a) Cycling stability of CCG/PANI nanofiber composite and PANI nanofiber films. Reprinted with permission from [88]. Copyright 2010 American Chemical Society. (b) Cycling performance of PPy nanofiber/GO nanocomposite and PPy nanofiber. Reprinted with permission from [89]. Copyright 2010 American Chemical Society. (c) Schematic illustration of PANI/CNT nanocomposite electrodes well solidified in the polymer gel electrolyte. (d) Cycle stability of PANI/CNT nanocomposite electrodes. Reprinted with permission from [91]. Copyright 2010 American Chemical Society.
Download figure:
Standard image High-resolution image3. Application of 1D nanomaterials in energy storage
3.1. Rechargeable batteries
As one of the most mature electrochemical energy storage systems, rechargeable batteries are widely used in our daily lives and are foreseen to be used in various fields. For example, LIBs have been extensively employed in personal electronic devices such as laptops, mobile phones, and so on [92]. Moreover, they have become increasingly prevalent as powertrains for electric vehicles and hybrid electric vehicles [93]. 1D nanomaterials have many special properties due to their small size and variety of different geometries. It is evident that various electrode materials with 1D nanostructures exhibit enhanced electrochemical performance. Recently, a large number of 1D nanomaterials have been reported for application in rechargeable batteries. In 2012, Lu's group [94] reported a strategy towards binder-free, high-rate electrodes through the formation of conformal TiO2 nanocrystal (NC) coatings on CNT scaffolds followed by a sintering process that removes the capping agents on the NCs, creating a conformal, mesoporous, TiO2 NC coating on CNTs. This electrode material showed a total capacity of 200, 170, and 110 mAh g−1 (based on the total weight of the material), respectively, at 0.25 C, 2 C and 20 C. In addition to their outstanding capacity and rate performance, these composite electrodes exhibited excellent cycling stability. The capacity decayed slowly at high rates (e.g. a capacity loss of 10 mAh g−1 for 200 cycles at 10 C, and 9 mAh g−1 for an additional 200 cycles at 20 C). When returning to 2 C, a stable capacity of 150 mAh g−1 was resumed and negligible capacity loss was observed after 100 additional cycles. It is well known that the hetero-atom-doping of carbon materials is an effective approach to enhance their Li+ storage performance. Sun et al [95] compared the Li+ intercalation/de-intercalation performance of CNTs doped with 16.4 at.% of nitrogen, synthesized using floating catalyst CVD, with that of pristine CNTs. The highly nitrogen-doped CNT (HN-CNTs) anode showed a much higher specific capacity (494 mAh g−1) than pristine CNTs (260 mAh g−1). In addition, HN-CNTs still delivered 397 mAh g−1 at the 100th cycle (only 266 mAh g−1 for CNTs). Moreover, the HN-CNTs displayed a better rate capability than the CNTs. The reason for the enhanced performance was attributed to the high concentration of nitrogen in the CNTs, which facilitated higher electrical conductivity. Also, doping nitrogen into CNTs led to more defect sites in the anodes, which provided more Li+ storage on electrochemical active sites.
Si-based anodes exhibit intrinsic high energy density with good capacity retention and reversibility. Hence, Kumta et al [96] synthesized novel 1D heterostructures comprising VA-CNTs containing nanoscale amorphous/nanocrystalline Si droplets, deposited directly on VA-CNTs with clearly defined spacing using a simple two-step liquid injection CVD process. It was demonstrated that the hybrid silicon/CNTs showed a high reversible capacity of 2000 mAh g−1 with very little fading in capacity of 0.15% per cycle over 25 cycles [96].
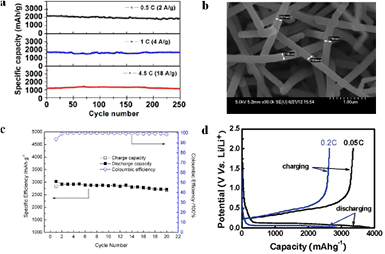
Silicon is regarded as one of the most promising anode materials for LIBs, so there are a considerable number of reports about its application. Zhou et al [97] manufactured porous doped SiNWs with large pore size and high porosity. Taking advantage of the pore structure relieving the stress in the Si materials upon cycling, the length of cycle retention was improved. Porous doped SiNWs showed high capacity (2000 mAh g−1) after 250 cycles at a 0.5 C rate (figure 6(a)). SiNWs with interconnected microstructure were prepared using the vapor–liquid–solid process using Al/Cu films as the catalyst [98]. These materials exhibit a high initial reversible capacity (2836 mAh g−1) at a 0.1 C rate, with an initial coulombic efficiency of 93.7%. After 20 cycles, the capacity remained at 2600 mAh g−1, i.e. without a large loss (figures 6(b) and (c)). Yang et al [99] made SiNWs using a Cu-catalyzed CVD method, showing a first cycle reversible capacity of 1640 mAh g−1. After 100 galvanostatic cycles the material still delivered a capacity of 1300 mAh g−1. In 2010, Paik et al [100] reported the preparation of arrayed, sealed SiNTs via a sacrificial template strategy. The SiNTA electrodes displayed a high initial capacity (3360 mAh g−1) and coulombic efficiency (87%) at a 0.05 C rate (figure 6(d)). The sealed geometry suppressed excessive electrolyte decomposition by reducing the interfacial area between the electrode and electrolyte, so both the initial coulombic efficiency and capacity retention were improved. In 2012, Lee et al [101] synthesized free-standing Si nanorods on a copper substrate by electron-cyclotron-resonance CVD. During the first cycle, the material presented a discharge capacity of 2911 mAh g−1 and a coulombic efficiency of 95%. The capacity retention was 84% after 25 cycles. Bundles of Si nanorods were also fabricated by electron-cyclotron-resonance plasma-enhanced CVD. At the first cycle, the material delivered a specific discharge capacity of 2990 mAh g−1 with a coulombic efficiency of 92%. The bundle-type Si nanorods had a capacity of 1420 mAh g−1 and a stable coulombic efficiency (higher than 97%) after 100 cycles. Park et al [102], inspired by the structural characteristics and working principles of sticky spider-weds, integrated γ-Fe2O3 particles with hierarchically porous, sticky, spider-web-like MWCNTs, which were synthesized through a process involving ozonation, ice-templating assembly, and thermal treatment. Serving as the LIB anode material, the composite web showed a high capacity (822 mAh g−1 at 0.05 A g−1), high rate capability (72.3% retention from 0.05 to 1 A g−1), excellent cycling stability (88% capacity retention after 310 cycles), and high coulombic efficiency (above 99%) [102]. Mai et al [103] proposed thermal induced strain relaxation of 1D iron oxide, designing a ladder-like α-Fe2O3 nanostructure which showed an outstanding reversible capacity of 1200 mAh g−1 at 100 mA g−1 and a significant high-rate cyclability with a capacity loss of 0.056% per cycle for 1200 cycles at 5 A g−1.
Figure 6. (a) Charge/discharge capacity of a porous SiNW anode at 0.5 C, 1 C, and 4.5 C for 250 cycles. Reprinted with permission from [97]. Copyright 2012 American Chemical Society. (b) SEM image of SiNWs. (c) The cyclability of a SiNW electrode for 20 cycles. Reprinted with permission from [98]. Copyright 2013 American Chemical Society. (d) Voltage profiles of arrays of sealed SiNTs for the first cycle at 0.05 C and 0.2 C. Reprinted with permission from [100]. Copyright 2010 American Chemical Society.
Download figure:
Standard image High-resolution image3.2. Supercapacitors
The enormous growth in electric vehicles and hybrid electric vehicles has facilitated the urgent and increasing demand for high-power energy resources. Owing to their high energy densities, rechargeable batteries are becoming the most common electrical energy-storage device. However, rechargeable batteries suffer from low power delivery. In contrast, supercapacitors can provide higher power densities, faster charge/discharge processes (within seconds), and a long cycle life (>105) [104, 105]. Nevertheless, the critical challenge for their use in practical applications is the increase of their energy density. For this purpose, electrodes with large capacitance are being developed [30, 106].
CNTs and nanocomposites have the advantages of high electrical conductivity and accessible surface area, for which reasons they have been tested as materials for supercapacitors. High capacitance values have been reported for CNT–PPy nanocomposites [107]. Because the internal and external surfaces of the MWNTs were coated with a thin PPy film during the electrochemical polymerization, the nanocomposites showed a capacity of nearly 130 F g−1 because the thin PPy layer offered fast pseudo-Faradaic processes. Zhang et al [108] reported porous nitrogen-doped CNTs (PNCNTs) derived from T-PPy. The PNCNTs showed a hierarchically porous nanostructure doped with nitrogen species. The hierarchical porous nanostructure allowed the rapid diffusion of electrolyte ions while the nitrogen doping improved the wettability of the PNCNT electrode. Overall, the PNCNTs showed high specific capacitance, excellent rate performance, and long-term cycling stability. At a current density of 0.5 A g−1, the material delivered a high specific capacitance of 210 F g−1.
Taking into account the low specific capacitance of CNTs, activated carbon-coated MWCNT doped with nitrogen (N-ACMWCNT) derived from PPy-coated MWCNTs were developed. The method allowed the fabrication of electrodes with high active material loadings, in the range of 15–35 mg cm−2, and a high active material to current collector mass ratio, in the range of 0.21–0.50. The N-ACMWCNTs showed excellent performance as electrodes for supercapacitors, with an energy density of 16.1 mWh g−1 and a power density of 14.4 W g−1 in a voltage window of 1.8 V. The supercapacitors also exhibited good capacitance retention at high charge/discharge rates, and good cycling stability [109].
Recently, 1D conducting polymers have been researched widely as supercapacitor electrode materials. Wei et al [110] synthesized oriented PANI NW arrays by a one-step, template-free method. The vertically aligned PANI NWs had higher capacitance values than disordered NWs thanks to their unique structure. The electrodes achieved 950 F g−1 as the highest capacitance while maintaining 780 F g−1 upon high current density (40 A g−1). Yu et al [111] fabricated graphene–PANI nanorod composite paper by a one-step reduction method. The material showed high capacitance (763 F g−1) at a current density of 1 A g−1 and kept 82% of its initial capacitance after 1000 cycles. Oliveira et al [112] explored CNT–PPy nanofibers core–shell composites. During polymerization of hollow PPyNTs, the combination with titanium dioxide nanoparticles and SWCNTs returned good charge storage performance in organic supercapacitors, with a specific capacitance of 282 F g−1.
Drzal et al [113] combined PPyNWs with conductive graphene nanosheets to improve capacitance and electronic conductivity. When applied in a supercapacitor, high ionic and electronic transport is achieved, resulting in a composite electrode specific capacitance of 165 F g−1 after 1000 electrochemical cycles at 1 A g−1. Yoon et al [114] investigated the electrochemical capacitive performance of PEDOT NTs with different surface substructures. The nanorods substructures of PEDOT NTs offered a capacitance of 153 F g−1 corresponding to 73% of the theoretical capacitance. What is more, there was an increase of 17% in specific capacitance when coupled to manganese dioxide. Lee et al [115] report a simple one-step method to synthesize MnO2/PEDOT coaxial NWs by co-electrodeposition in a porous alumina template. The coaxial NWs not only exhibit high specific capacitance but also demonstrate outstanding capacitance retention even at high current density. In fact, the electrodes of such a material preserved 85% of their specific capacitance (from 210 to 185 F g−1) when the current density increased from 5 to 25 mA cm−2.
4. Summary and outlook
Over the past two decades, 1D nanomaterials have attracted growing research interest due to their large length-to-diameter ratio, fascinating electrical, mechanical, chemical properties, and widespread potential applications. This review of both the synthesis and the applications of 1D nanomaterials indicates how these materials may overcome the limitations of current electrode materials to realize the ultimate goals of higher capacity, faster charge and discharge performance, and higher cycle life stability.
Amongst the various structures for rechargeable battery electrodes, 1D nanostructures with much shorter bi-continuous ion and electron transport pathways demonstrate the advantages of high-rate performance. Moreover, their broader surface area provides easier access of the electrolyte to the redox active material, while their small size facilitates stress relaxation, boosting their capacity of accommodating the volume changes associated with electrochemical reactions.
As an additional consequence of the broader specific surface, 1D nanostructures exhibit extraordinary charge storage performance via double layer or fast pseudocapacitive surface redox processes for supercapacitor applications. Electrodes with 1D nanostructures enable bi-continuous transport of ions and electrons, which is beneficial for fabricating high energy density hybrid supercapacitors.
Although immense progress has been achieved in both the synthesis and application of 1D nanomaterials, almost unlimited research opportunities are being and will be explored by countless laboratories around the world. Nevertheless, efforts are still required in order to satisfy the requirements of industrial applications. These efforts should be focused on the primary challenges, such as synthesis scale, production cost, safety problems, and environmental issues.
This review, discussing the novel design and controllable synthesis methods of 1D nanomaterials, shows that more in-depth understanding of the current synthetic strategies is desirable. The development of more robust synthesis methods enabling the production of 1D nanomaterials at a large scale and in an environmentally friendly and cost-effective manner need to be identified. However, this review may provide an avenue for more sophisticated design and synthesis of 1D nanomaterials and stimulate greater interest and efforts towards their functionalization and applications.
Acknowledgments
This work was supported by the National Natural Science Fund for Distinguished Young Scholars (51425204), the National Natural Science Foundation of China (51521001), the National Key Research and Development Program of China (2016YFA0202603), the Programme of Introducing Talents of Discipline to Universities (B17034), the International Science and Technology Cooperation Program of China (2013DFA50840) and the Fundamental Research Funds for the Central Universities (WUT: 2016III001, 2017IVA096, 2017III009, 2017III006). LM gratefully acknowledges financial support from China Scholarship Council (No. 201606955096).