Abstract
Chemotherapy is the primary option for the treatment of cancer, inflammation, and infectious diseases. Conventional drug delivery poses solubility and bioavailability challenges, systemic toxicity, non-specific targeting, and poor accumulation of chemotherapeutic drugs at the desired site. Nanotechnology has led to the development of various nanomaterials that have decreased the toxicity and increased the accumulation of drugs at the target site. Systemic administration of nanomaterials causes burst release and non-specific targeting of chemotherapeutics, leading to off-target organ toxicity. Drug delivery based on low molecular weight hydrogels (LMWHs) provides a suitable alternative for drug delivery due to their ability to entrap chemotherapeutic drugs. Injectable and biodegradable LMWHs allow the administration of chemotherapeutics with minimal invasion, allow the sustained release of chemotherapeutic drugs for long periods, and reduce the challenges of immunogenicity and low drug entrapment efficiency. Herein, we summarize the advances in the engineering of LMWHs for controlled and prolonged delivery of chemotherapeutics for cancer, infectious diseases, and inflammatory disorders.
Export citation and abstract BibTeX RIS
1. Introduction to challenges of chemotherapy
Chemotherapy is extensively used for the treatment of many chronic and infectious diseases. Antimicrobial chemotherapy helps mitigate the microbes responsible for infectious diseases, anticancer chemotherapy inhibits the proliferation of cancer cells, and anti-inflammatory chemotherapy diminishes the uncontrolled inflammation. Chemotherapeutic drugs are usually administered through conventional systemic routes of intravenous injection or oral ingestion. Therefore, chemotherapeutic drugs' solubility and stability is the first critical challenge that reduces the efficacy of chemotherapeutic drugs. Although convenient, oral delivery offers a limited bioavailability in the blood due to numerous gastrointestinal (GIT) barriers such as extreme pH conditions, gastric enzymes, microbiota, and low GIT absorption [1]. Intravenous delivery of chemotherapeutic drugs enhances the bioavailability of drugs in the blood and is highly encouraged for water-soluble drugs. In contrast, delivery of poorly water-soluble drugs is challenging as these drugs are prone to precipitation and can cause serious toxic effects. Therefore, excipient molecules are usually used to solubilize the hydrophobic drugs like docetaxel, an anticancer drug, administered in cancer patients as a suspension in polysorbate 80 [2]. Similarly, oral suspension of amphotericin B, an antifungal medication, contains sodium saccharin as an excipient [3].
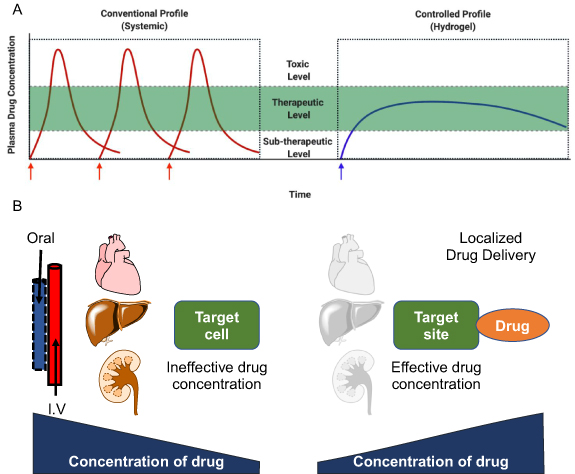
Pharmacokinetic profiling and biodistribution determine the efficacy of chemotherapeutic drugs upon delivery as the drug concentration in blood circulation diminishes over time. A study in rats demonstrated that peak plasma concentration of doxorubicin, an anticancer drug, decreased ~5.5 times within an hour [4]. Flucytosine, an antifungal medication, is recommended to be used four times a day due to its very short half-life [5]. Poor circulation and rapid clearance of chemotherapeutic drugs lower drugs' concentration at the target site [6]. Therefore, maintaining the therapeutic concentration of drugs in the blood plasma is critical for its effective action. Most chemotherapeutic drugs have a narrow therapeutic index, a range of dose required for the desired therapeutic response and toxicity. Conventional systemic administration of chemotherapeutic drugs is inefficient in delivering the desired therapeutic concentration at the target site due to the narrow therapeutic window [7]. Therefore, multiple doses of chemotherapeutic drugs are usually administered to maintain the drug's therapeutic levels in blood circulation (figure 1(A)). Poor patient compliance adhering to this chemotherapy regime leads to ineffective treatment and drug resistance, as seen in case of tuberculosis treatment.
Figure 1. Challenges of chemotherapy treatments. (A) Plasma drug concentration profile for conventional drug delivery and sustained or controlled drug delivery vehicles. (B) Schema showing the change in drug concentration from the injection site to the target for traditional (intravenous or oral) and localized drug delivery systems (created with BioRender.com).
Download figure:
Standard image High-resolution imageChemotherapeutic drugs are intended to kill tumor cells. However, they also kill actively growing cells in the body, like cells in the bone marrow, hair follicles, GIT, reproductive system, and nervous system, therefore leading to several side effects like fatigue, abnormal bleeding, hair loss, nausea, vomiting, erratic bowel movement and numbness in limbs. Systemic circulation of chemotherapeutic drugs causes severe cytotoxicity due to accumulation at non-target sites resulting in tissue damage in various organs like the heart, kidney, lungs, and nervous system. Medication-induced damage to vital orangs such as the heart (cardiotoxicity), liver (hepatotoxicity), and kidneys (nephrotoxicity) majorly affects overall survival or quality of life in patients.
In conventional treatment, systemic delivery of antibiotics can clear the infection. However, it is associated with undesired side effects like amphotericin B causes renal toxicity by direct tubular damage [8], and flucytosine may lead to bone marrow suppression [5]. A combination of antituberculosis drugs taken orally for 6–8 months can cause severe side-effects of ototoxicity, hepatoxicity, vision impairment, and gastric upsets [9, 10]. Similarly, anticancer drugs like doxorubicin administration is associated with cardiotoxicity [11], whereas platinum-based drugs like cisplatin, carboplatin, and oxaliplatin cause nephrotoxicity, myelosuppression, and neurotoxicity [12], and taxanes like paclitaxel can induce myelosuppression and peripheral neuropathy [13]. Therefore, solubilization, stability, long circulation, reduced accumulation at the non-specific target site, and maintaining the drug concentrations at the target site are required for effective chemotherapeutic treatments (figure 1(B)).
2. Drug delivery systems
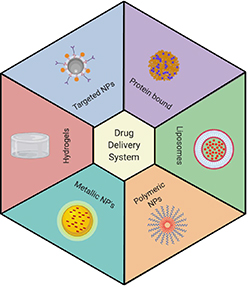
Challenges associated with chemotherapeutic drugs have been addressed by engineering numerous drug delivery systems (DDS) that can protect the drug and transport the pharmaceutically active compounds in the body to achieve the desired therapeutic effect at the target site (figure 2). DDS can also determine the rate as well as the location for the release of a drug. A variety of DDS have been employed to improve the therapeutic efficacy of drugs with reduced toxicity by increasing the bioavailability of drugs, enhancing the half-life in blood circulation, enhancing the stability of drugs in the body, reducing the excretion from kidneys, decreasing the interactions with off-target sites in the body, and achieving controlled release of drugs (figure 2) [14, 15].
Figure 2. Drug delivery systems. Various drug delivery systems comprise protein-bound nanoparticles, liposomes, targeted nanoparticles, polymeric nanoparticles, gold and silver nanoparticles, and hydrogels (created with BioRender.com).
Download figure:
Standard image High-resolution imageNanoformulations in the nanometer size range (1–100 nm) exhibit different physical and chemicals properties than their micro or macro size counterparts [16]. Nanoformulations can solubilize the insoluble drugs, like paclitaxel administrated in a nanoparticulate form like albumin-bound paclitaxel (Abraxane), or can solubilize the water-soluble drugs like doxorubicin in an aqueous compartment of liposomes, and are being used for the treatment of various solid tumors. Tumor vasculature is abnormal and leaky that facilitates the accumulation of nanoparticles (NPs), and improves the biodistribution with lesser adverse effects than the parent drug. Therefore, enhanced permeation and retention effect allows the preferential accumulation of NPs at the tumor site, called passive targeting of NPs [17]. Surface modification of NPs using PEGylation prevents the phagocytosis of NPs, prolong the systemic circulation, and increase the plasma half-life of drugs [18]. PEGylated liposomal formulation of doxorubicin (DOXIL®), the first FDA-approved nanoformulation, does not allow its interactions with immune components, and helps in prolonged circulation, therefore, increases its accumulation at the target tumor site [19, 20]. Liposomal formulation of amphotericin B has improved its solubility and achieved reduced toxicity [21].
Active targeting of drugs can be achieved by tethering antibodies, peptides, and small molecules specific for the receptors on the surface of NPs [22]. Triple-negative breast cancer (TNBC) does not respond to hormonal therapy due to the absence of hormonal receptors on the tumor cell surfaces. Angiopep-2 peptide conjugated on the surface of DOX-DGL- gelating NPs increased the accumulation of NPs around the TNBC through passive and active tumor targeting [23]. Treatment of brain cancer is challenging due to the low permeability of chemotherapeutic across the blood-brain barrier. Angiopep-2 can cross the blood-brain barrier by receptor-mediated transport, and when conjugated with the anti-VEGF monoclonal antibody, can actively target glioma cells in the brain [24]. PLGA-PEG-based polymer NPs conjugated to RNA aptamer showed targeted delivery of docetaxel in a prostate cancer model [25]. Similarly, biotinylated antibody-conjugated polymeric micelles of daunomycin had shown enhanced accumulation in the brain that can be employed for brain targeting [26]. PEGylated interferon-alpha 2a sold under brand name Pegasys is an anti-hepatitis B virus therapy to attain sustained virologic response in patients with chronic hepatitis B virus infection [27].
Apart from the advantages of NPs in cancer therapy, NPs have been shown to enhance the effect of antibiotics and anti-inflammatory drugs. Gold NPs conjugated with streptomycin showed enhanced efficacy against Gram-positive and Gram-negative bacteria [28]. Gold NPs conjugated with fluoroquinolone showed synergy in combating drug-resistant Escherichia coli infections as well [29]. NPs have also been employed for drug delivery as a coating on implantable devices and wound dressings for better antimicrobial efficacy and wound healing [30]. Polymeric NPs modified with vasculature targeting ligands on their surface can comprehend inflammation-directed drug delivery [31]. Proteolipid vesicles consist of leukocyte plasma membrane proteins incorporated in lipid NPs. The leukosomes (proteolipid) retained the flexibility and physicochemical properties of liposomal formulations along with active targeting of inflamed vasculature by leukocyte membrane proteins. Dexamethasone, an anti-inflammatory glucocorticoid, loaded leukosomes showed selective and active targeting of NPs to inflamed tissue. The effective delivery of dexamethasone reduced inflammation in the localized ear inflammation model in mice [32].
3. Hydrogels for drug delivery
Hydrogels are supramolecular aggregates of small molecules or polymers held together by electrostatic, hydrophobic, van der Waals forces, and hydrogen-bonding interactions and have high water loading capacity [33]. Hydrogels also use these interactions to hold a wide range of chemotherapeutic drugs with varied hydrophobicity and hydrophilicity. Polymeric hydrogels can hold a large amount of water, and therefore, can entrap chemotherapeutics in their hydrophilic cross-linked networks with a controlled drug release profile [34]. Natural polymers like cellulose, alginate, chitosan, hyaluronic acid, collagen, heparin, and gelatin are biodegradable that aids in the sustained release of cargo upon degradation [35]. Catechol derivatization of chitosan forms hydrogel by strong and reversible coordinative interactions upon addition of Fe(III) ions. The polymeric hydrogel is biocompatible and stays for more than 40 d in mice. The catechol-chitosan (CAT-CHIT) hydrogel can entrap a combination of hydrophobic (docetaxel) and hydrophilic (doxorubicin) drugs and can maintain their sustained release for 18 d. Localized injection of a combination of drugs encapsulated in CAT-CHIT hydrogel showed a significant decrease in LLC tumor volume than the injection of drugs without hydrogel near the tumor site [36]. Numerous polymers based on different scaffolds have been explored for delivery applications [37].
Low molecular weight hydrogels (LMWHs) are small amphiphilic molecules that can self-assemble into a three-dimensional structure using non-covalent interactions and encapsulate a large amount of water. Non-covalent interactions, such as electrostatic interactions, H-bonding, hydrophobic interactions, and van der Waals interactions, are responsible for the hydrogelation of amphiphilic molecules [38]. Ability of hydrogels to encapsulate chemotherapeutic drugs is contingent on amphiphilicity of hydrogelator, including polar and non-polar (hydrophobic) scaffolds. Delivery of chemotherapeutics using hydrogels can be easily controlled by pore-size, degradation rates of hydrogels, the charge of polar head groups, and hydrophilicity of backbone. Dibenzoyl-L-cystine was the first small-molecular hydrogel reported by Hoffman [39]. Authors dissolved dibenzoyl-L-cystine in 95% alcohol, boiled, and then added water to make the gel. As the process of hydrogenation is dependent on solubilization followed by supramolecular interactions of amphiphilic molecules, hydrophobicity and hydrophilicity has been tuned on numerous amphiphilic molecules to allow them to self-assemble using hydrophobic and electrostatic interactions [40]. Conventional amphiphiles comprise a polar head group like carboxylic acid, amino acids, sugars, phosphate, or quaternary amine groups, whereas hydrophobic groups can vary from long alkyl chains to steroidal moieties [41]. As these amphiphiles are not soluble in water as such, suspension of the amphiphiles can allow these amphiphilic molecules to self-assemble in supramolecular aggregates like micelles, liposomes, bilayers. A critical concentration of amphiphiles is needed to form hydrogels where these amphiphiles aggregate and entrap large amounts of water, and possess viscoelastic behavior. Chemical modifications at the polar and non-polar scaffolds allow engineering of numerous hydrogels that are reversible in nature, and has been explored for various biomedical applications.
LMWHs have advantages for drug delivery applications due to their uncomplicated synthesis and ease of modulating the supramolecular interactions between amphiphilic molecules. LMWHs can be easily degraded biologically due to amphiphiles' small molecular nature and can be easily excreted out of the body, thereby avoiding surgical removal [42]. In addition, the release of chemotherapeutics from LMWHs can be controlled by fine-tuning the supramolecular interactions by incorporating pH, temperature, and enzyme sensitive moieties in the gelator [43]. Ability of LMWHs to entrap hydrophobic and hydrophilic molecules can allow the delivery of a combination of drugs, antibiotics, DNA, and RNA, and release them in a sustained manner at the targeted site.
4. LMWHs for cancer therapy
Despite preclinical validation of different NP-based therapeutics for cancer therapy, non-specific distribution and inadequate accumulation of the drug in tumor tissue remain a major challenge apart from limited drug loading capacity and burst release of drugs from nanoformulations in the systemic circulation. As most chemotherapeutic treatment regimens use a combination of drugs, encapsulation of a combination of drugs in NPs with tumor targeting, and maintaining the sustained drug release profile is difficult to achieve. Induction of immune response on systemic delivery of nanotherapeutics further lowers their efficacy leading to off-target side effects [44]. Therefore, the concept of the controlled release of drugs originated around 1950 with the introduction of an external infusion pump that lowered the adverse effects on the hematopoietic system compared to intravenous administration [45]. Localized drug delivery involves delivering chemotherapeutics at the targeted site, allowing the chemotherapeutic drugs to target the disease site without entering the bloodstream. It also aims to enhance the concentration of active ingredients at the diseased site by employing various polymeric wafers, polymeric hydrogels, and LMWHs [46–48]. LMWHs have the edge over the use of systemic delivery of NPs for cancer chemotherapy as it allows the delivery of chemotherapeutics at the diseased site. Numerous advantages of exploring LMWHS for cancer therapy include easy synthesis and biodegradable nature, ability to module the viscoelastic behavior for modulating the drug release, no interactions with reticuloendothelial system, and sustained release of combination of drugs at target site. We have organized the LMWHs for caner therapy into two categories based on the entrapment or conjugation strategy of drugs.
4.1. Drug conjugated LMWHs for anticancer therapy
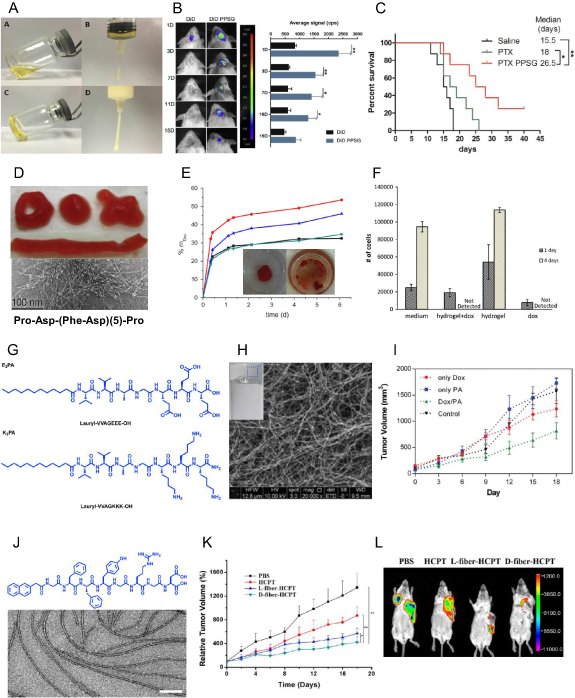
Prodrug conjugation strategies using tethering of anticancer drugs to gelators can allow the sustained release of chemotherapeutic drugs. They will not allow burst diffusion of the drug in systemic circulation as observed in drug-encapsulated nanocarriers. Conjugation of hydrophobic drugs like paclitaxel and folic acid to phosphorylated tripeptide (GpYK) helps in assembly of these molecules into hydrogels (figure 3(A)). Upon adding phosphatase, FA-GpYK-Taxol (4% w/v) solution in PBS gets converted to FA-GYK-Taxol in 3 min and self-assembles into the nanosphere (figures 3(B) and (C)). Anticancer evaluation of these nanospheres in HepG2 cells showed similar IC50 values as that of taxol itself. As FA-GYK-Taxol hydrogel has a higher weight percentage of taxol (49%) than liposomal or taxol-polymeric systems [49], this study provides a better solution for solubilization, entrapment, and delivery of hydrophobic drugs. Folic acid-peptide-taxol conjugates have folic acid as a targeting ligand, and these conjugates can self-assemble to form hydrogels by glutathione-mediated disulfide bond reduction (figure 3(D)). Hydrolysis of ester bond facilitated the sustained release of taxol from the hydrogel. A single intratumoral injection of hydrogel into 4T1 tumor-bearing mice inhibits the tumor growth more efficiently than intravenous administration of taxol (figure 3(E)) [50].
Figure 3. Drug conjugated LMWHs for anticancer therapy. (A) Molecular structure of FA-GpYK-Taxol. (B) Dynamic time sweep frequency graph upon phosphatase addition confirms the gel's injectable nature (insert: the image of gel). (C) Atomic force micrograph of FA-GYK-Taxol gel suggesting its nanosphere nature. (D) Molecular structure of Folic acid (FA) peptide-Taxol conjugate and image of the hydrogel. (E) Relative tumor growth kinetics of xenografted mouse breast tumor (4T1-luciferase) upon treatment with Folic acid (FA) peptide-Taxol hydrogel shows the reduction in tumor growth kinetics. (F) Molecular structure of amphiphilic peptide conjugated to platinum and image of the hydrogel. (G) Cumulative release of the Pt(II) drug from hydrogel shows glutathione (GSH) mediated release of drug from hydrogel matrix. (H) Relative tumor growth kinetics of mouse 4T1 breast tumor confirms the reduction in tumor volumes upon treatment with hydrogel (1). (I) Biodistribution profile of the platinum drug in tumor and other major organs in 4T1 breast tumor upon treatment with hydrogel (1) shows an enhanced accumulation of the drug in hydrogel-mediated delivery. (J) Molecular structure of curcumin pro-gelator Cur-FFE-ss-ERGD and its gelation catalyzed by glutathione (GSH). (K) Tumor volume graph of 4T1 luciferase xenograft treated with curcumin pro-gelator and Cur-hydrogel shows the reduction in tumor growth. (L) Molecular structure of Taxol-SA-GSSG molecule. (M) TEM image of taxol hydrogel in PBS (scale bar 200 nm). (N) Bioluminescent images of 4T1 luciferase xenograft show a reduction in tumor volumes on treatment with taxol-hydrogel (figures 3(B)–(E) are adapted/modified from [49] and [50] with permission from Royal Society of Chemistry, copyright 2011 and 2013; figures 3(F)–(I) from [51] with permission from Royal Society of Chemistry, copyright 2014; figure 3(K) from [52] with permission from Royal Society of Chemistry, copyright 2014; and figures 3(M)–(N) from [53] with permission from Elsevier, copyright 2012).
Download figure:
Standard image High-resolution imageEnzyme-mediated release of drugs conjugated to self-assembled amphiphiles can provide a better strategy for the controlled release of chemotherapeutic drugs. An amphiphilic peptide conjugated to platinum drug undergoes supramolecular self-assembly to form the hydrogel by undergoing dephosphorylation mediated by Alkaline phosphatase (figure 3(F)). Glutathione (GSH) mediated reduction of hydrogel-platinum conjugate releases the platinum drug in a controlled manner (figure 3(G)). Localized and sustained release of platinum drug from prodrug gets accumulated in the 4T1 tumors and showed significant anticancer activity as evident from tumor growth kinetics (figure 3(H)). All the major organs, including kidney and liver, showed reduced accumulation compared to free cisplatin with increased uptake of platinum by tumor tissue as compared to control mice (figure 3(I)) [51].
Glutathione (GSH) responsive peptide conjugated to a chemotherapeutic drug allowed the drug-peptide conjugate to have improved water solubility in an aqueous buffer. Curcumin has been explored for broad-spectrum biomedical applications, but its use is limited due to low aqueous solubility. Curcumin conjugated to RGD peptide having disulfide linkage (Cur-FFE-ss-ERGD) showed enhanced aqueous solubility (figure 3(J)). The progelator solubilized in PBS formed yellowish hydrogel upon reduction with GSH. The curcumin pro-gelator showed superior anticancer activity in the 4T1-luciferase breast cancer model compared to the nanofibrous hydrogel and systemic injection of curcumin (figure 3(K)) [52]. It has also been shown that oxidized glutathione (GSSH) conjugated to paclitaxel via succinic acid (SA) linker (Taxol-SA-GSSG) enhances the aqueous solubility of taxol (figure 3(L)) [53]. Taxol-conjugate self-assembles to form a nanofibrous hydrogel upon hydrolysis of the ester bond between paclitaxel and SA (figure 3(M)), which showed significant anticancer efficacy against 4T1-luciferase tumor with reduced metastasis compared to clinically useful intravenous formulation of taxol (figure 3(N)). Low blood concentration of taxol with improved tumor growth inhibition compared to free paclitaxel witnessed the excellent potential for hydrogel-mediated localized chemotherapy.
4.2. Drug encapsulating LMWHs for anticancer therapy
A large number of LMWH based on peptides have been used to entrap anticancer drugs like doxorubicin [54], paclitaxel [49], and camptothecin [55], as entrapment strategy allows the loading of the combination of drugs without any chemical modification in the hydrogel structure. A 16 amino acid-based peptide self-assemble and form nanofibrous structures can encapsulate ellipticine, an alkaloid that inhibits DNA Topoisomerase II, with better solubility and stable rheological properties [56]. Similarly, a mixture of natural phospholipids, triglycerides, and ethanol has been shown to self-assemble into a biodegradable hydrogel that can entrap paclitaxel (figure 4(A)) and maintain a sustained release upon intracranial implantation in the mouse brain over a prolonged period (figure 4(B)). Paclitaxel-loaded hydrogel showed enhanced therapeutic efficacy in glioma-bearing mice upon intracranial injection (figure 4(C)) [57].
Figure 4. Drug encapsulating LMWHs for anticancer therapy. (A) Morphology of paclitaxel loaded phospholipid based gel (PG) before and after gelation. (B) In vivo images of mice and their quantification after injection of free DiR and DiR loaded PG showing the sustained release of the dye. (C) The percentage survival graph of glioma tumor-bearing mice confirms the increase in mice survival on treatment with PTX-PG gel. (D) Optical image and TEM image of DOX loaded PFD-5 hydrogel. (E) Drug release assay of DOX from peptide hydrogel (5%) loaded with 0.40 mg (green), 0.24 mg (blue), 0.14 mg (red) of DOX, and from peptide hydrogel (5.2% w/v) loaded with 0.14 mg (black) of DOX witnessing dose-dependent release of DOX. (F) Cell viability assay shows the increase in cell death after treatment of SaOS2 cells with DOX loaded PFD-5 hydrogel. (G) Molecular structure of E3PA and K3PA molecule that self-assemble to form the hydrogel. (H) SEM image of PA hydrogel and optical image of the gel. (I) Tumor growth kinetics of 4T1 tumor-bearing mice shows a reduction in growth kinetics upon treatment with DOX/PA hydrogel. (J) Molecular structure of Nap- GDFDFDYGRGD peptide and TEM image of peptide amphiphile (PA) hydrogel encapsulating hydroxycamptothecin (HCPT) (scale bar 100 nm). (K), (L) Tumor growth kinetics of 4T1 luciferase xenograft tumors (K) and bioluminescent images (L) after treatment with HCPT loaded D-fiber hydrogel confirm a significant decrease in tumor progression on treatment (figures 4(A)–(C) are adapted/modified from [57] with permission from Elsevier, copyright 2017; figures 3(D)–(F) from [58] with permission from Elsevier, copyright 2011; figures 3(H)–(I) from [59] with permission from Royal Society of Chemistry, copyright 2017; and figures 3(J)–(L) from [60] with permission from American Chemical Society, copyright 2014).
Download figure:
Standard image High-resolution imageAmong peptide scaffolds, Pro-Asp-(Phe-Asp)(5)-Pro (PFD-5) can form a monolayer in PBS and a β-sheet structure at physiological pH. The PFD-5 hydrogel can entrap doxorubicin (DOX), an active anticancer drug, upon the addition of CaCl2 (figure 4(D)). DOX-loaded PFD-5 hydrogel showed controlled release with 20%–40% DOX release within 24 h and 50% in 6 d (figure 4(E)). In vitro cytotoxicity assay showed the same efficacy as that of free doxorubicin (figure 4(F)) [58]. Peptide amphiphiles (PA) Lauryl-VVAGEEE and Lauryl-VVAGKKK-Am can self-assemble into supramolecular nanofiber structured hydrogel upon mixing in a 3:4 ratio (figures 4(G) and (H)). Biodegradability of PA hydrogel was determined by proteolytic degradation using proteinase K and α-chymotrypsin, resulting in 90% and 50% degradation in hydrogel mass in 25 d, respectively, compared to the buffer control that remained intact. Efficacy of DOX-encapsulated PA hydrogel (DOX/PA) against 4T1 cells showed higher toxicity than free DOX control. Similarly, anticancer efficacy against the subcutaneous 4T1 tumor model showed a significant reduction in tumor growth kinetics for localized injection of DOX/PA hydrogel than free DOX and PBS control (figure 4(I)) [59]. As proteolytic degradation of peptides based on L-aminoacids by protease can cause a burst release of drugs, Liu et al synthesized a D-amino acid-based peptide (Nap-GDFDFDYGRGD) that self assembles to supramolecular structure in PBS (figure 4(J)), and can maintain a sustained release of drugs for a more extended period. This D-amino acid based supramolecular construction is resistant against proteolytic degradation and serves as a suitable sustained drug delivery vehicle. Entrapment of hydroxycamptothecin (HCPT) in the peptide solution allowed the formation of nanofiber structured hydrogel (D-fiber-HCPT) (figure 4(J)). The D-fiber-HCPT significantly increased the efficacy of HCPT drugs in the 4T1 luciferase tumor model as compared to free HCPT and PBS control as evident by tumor growth kinetics graph (figure 4(K)) and in vivo bioluminescent images of treated and control mice (figure 4(L)) [60]. Our group has synthesized an L-alanine derivative of hydrazine (ALA-HYD) that can self-assemble into LMWH. ALA-HYD hydrogel can encapsulate doxorubicin with good rheological gel strength and injectability. DOX-Gel showed sustained release of the drug in PBS with ~40% release of DOX in 96 h. DOX-Gel exhibited higher anticancer activity against 4T1 tumors upon localized injection near the tumor site. Slow and continuous release of DOX from DOX-Gel showed significant regression in tumor volume as compared to intravenous injection and tumor site injection of free doxorubicin [61].
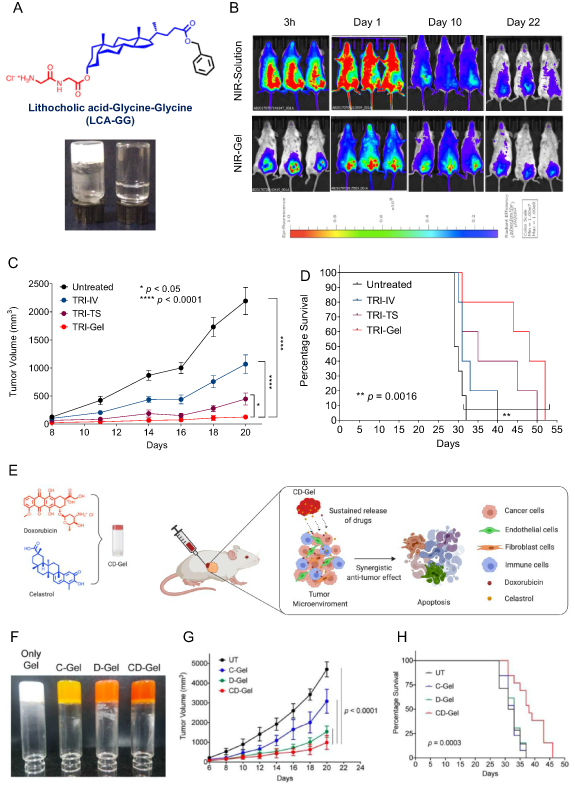
Recently, we reported the synthesis of a library of dipeptide based lithocholic acid (LCA) derived amphiphiles. We selected LCA-Gly-Gly amphiphile after screening and characterization of amphiphiles for their hydrogelation properties (figure 5(A)). The release profile of a model hydrophilic dye (NIR-820) encapsulated into this hydrogel showed localized and continuous release of the dye for more than 20 d after subcutaneous injection in mice (figure 5(B)). Next, we encapsulated a combination of doxorubicin (DOX), dexamethasone (DEX), and combretastatin A4 (CA4) in the gel (TRI-Gel) for combating the active proliferation of tumor cells, chronic inflammation, and angiogenesis in the tumor microenvironment, and compared the efficacy of TRI-Gel with localized tumor site (TRI-TS) and systemic delivery (TRI-IV) on tumor regression in the lewis lung carcinoma (LLC) model. TRI-Gel treatment showed significant retardation in tumor growth over other groups (figure 5(C)) with an increase in mice's median survival by 18 d (figure 5(D)) [62]. We also used LCA-Gly-Gly hydrogel for delivering the combination of celastrol (CEL), a triterpenoid, and an anticancer drug doxorubicin (DOX) (figures 5(E) and (F)). Upon screening different CEL and DOX drug concentrations, a synergistic effect on colon cancer cells was seen at a 1:1 ratio. Entrapment of CEL and DOX in hydrogel shows continued release of CEL and DOX from CD-Gel. CD-Gel showed higher efficacy on tumor regression with an increase in median survival in CT-26 tumor-bearing mice (figures 5(G) and (H)). CEL–DOX combination showed increased levels of ceramide and ceramide synthases in tumor tissue responsible for increased apoptosis, validated by lipidomics and gene expression data [63].
Figure 5. (A) Molecular structure of LCA-GG amphiphile that forms a hydrogel in water. (B) Fluorescence imaging of mice injected with IR-820 dye loaded hydrogel or dye alone without hydrogel on different days witnessing the localized and sustained release of the dye near the injection site. (C), (D) Tumor growth kinetics (C) and survival graph (D) of LLC tumor-bearing mice on different treatments show the enhanced efficacy of TRI-Gel over other treatments in reducing the tumor growth where TRI-Gel means hydrogel-mediated delivery of a combination of doxorubicin, combretastatin, and dexamethasone. TRI-TS and TRI-IV treatment are tumor site injection and systemic administration of the combination of drugs without hydrogel, respectively. (E) Schema showing hydrogel-mediated delivery of a combination of celastrol (CEL) and doxorubicin (DOX) for effective tumor regression. (F) Pictures of neat gel, CEL-Gel (C-Gel), DOX-Gel (D-Gel), and CEL and DOX (CD-Gel). (G), (H) Tumor growth kinetics (G) and survival graph (H) of CT26 tumor-bearing mice on different treatments showing synergistic effect CD-Gel treatment in tumor regression with an increase in mice survival (figures 5(A)–(D) are adapted/modified from [62] with permission from American Chemical Society, copyright 2019; and figures 5(E)–(H) from [63] with permission from Royal Society of Chemistry, copyright 2020).
Download figure:
Standard image High-resolution imageTherefore, these literature studies showed that LMWHs could provide an efficient and effective drug delivery platform, and their biocompatible, biodegradable, and non-immunogenic nature makes them an ideal delivery system. The engineering of drug-encapsulated hydrogels may not be advantageous as drug release kinetics from these hydrogels can only be modulated by changing hydrogels' chemical and physical properties. In contrast, drug-conjugated hydrogels provide the advantages where drug release kinetic parameters can be controlled through different chemical linkages between the drug and the hydrogelator. However, the design of a suitable scaffold that can self-assemble to form hydrogel is highly challenging. The use of stimuli-responsive linkages can modulate the degradation kinetics and drug release properties of these hydrogels. The injectable hydrogels can directly deliver drugs inside the tumor or adjacent to the tumor with minimal invasion or without the need for surgery. Therefore, LMWHs can significantly improve chemotherapeutics' efficacy and can be a better choice for cancer treatment in the future.
5. LMWHs for combating infectious diseases
Bacterial infections pose a severe threat due to the ability of microbes to challenge the body's immunity and its own ability to mutate themselves, making the antibiotics ineffective. Treatment strategies for these infections rely on a combination of antimicrobials that can kill or inhibit microbes' growth [64]. Proper and systematic use of antibiotics helps to clear infections and treat the disease, whereas inappropriate use of antibiotics can allow the bacteria to develop drug resistance, making them ineffective. Systemic delivery of antibiotics for combating wound infections is an ineffective strategy as biological barriers do not allow the delivery of antibiotics at an infected site in required concentrations, and high doses of antibiotics can cause undesirable side effects on vital organs [65]. Dissolution of poorly soluble antibiotics requires the use of various excipients that further imposes toxicity and irritations [66]. Therefore, the development of antimicrobial peptides (AMPs) [67], dextran [68, 69], and chitosan-based dressings [70] and metal NPs [71, 72] offer more occlusive treatment strategies. These strategies are challenged by low drug entrapment efficiency in existing formulations, their burst release, and the instability of loaded antibiotics to reach systemic circulation in effective concentration, thereby making them less effective [73]. In order to enhance the loading of antibiotics and their dermal penetration, NP-based strategies have been designed for topical applications [74]. But antibiotic-loaded hydrogels are more convenient due to their high entrapment efficacy and ability to maintain a sustained release of antibiotics [75]. Therefore, there is a need for drug delivery vehicles for antibiotics that can decrease the off-target toxicities and resistance abiding phenotypes with enhanced antibacterial efficacy.
5.1. Drug conjugated LMWHs for combating infectious diseases
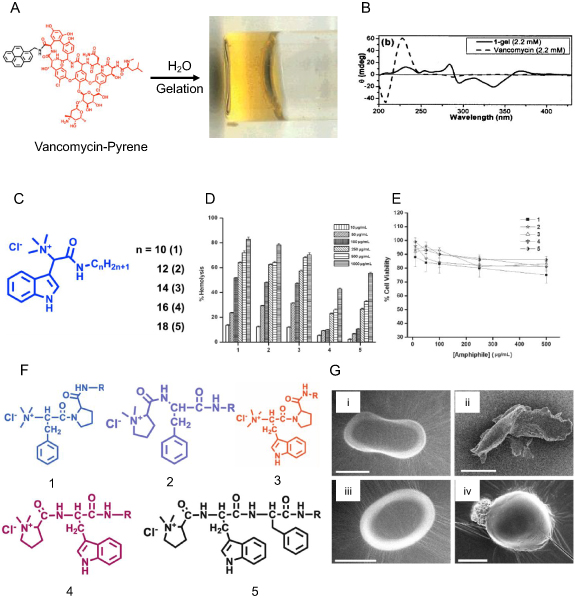
Vancomycin is an effective antibiotic for Staphylococcus aureus infections, but its use is limited due to low bioavailability. Rapid excretion of vancomycin by kidneys, and associated nephrotoxicity, discourages its systemic use [76]. Xing et al reported first antibiotic gelator where they tethered pyrene to C-terminal backbone of vancomycin to get vancomycin-pyrene. Conjugation of pyrene provided aromatic interactions and intermolecular hydrogen bonding in water by vancomycin provided the required interactions for hydrogel formation (figure 6(A)). Circular dichroism (figure 6(B)) and electron microscopy of gel suggested helical self-assembly of amphiphiles during hydrogel formation. Antibacterial experiments showed that hydrogel was potent at a concentration of 0.125–2 µgml−1, an 8–10 fold diluted concentration of vancomycin itself, against different vancomycin-resistant species of Enterococcus faecalis and Enterococcus faecium [77].
Figure 6. Drug conjugated LMWHs for combating infectious diseases. (A) Structure and pictorial representation of vancomycin pyrene-based (Van-pyrene) hydrogel. (B) Circular dichroism spectra of an aqueous solution of Van (2.2 mM) and Van-pyrene (1) hydrogel (2.2 mM). (C) Schematic structure of L-tryptophan based amphiphiles with varying alkyl chain length. (C), (D) Hemolytic assay (D) on human red blood cells and cell viability assay (E) of cationic amphiphile on the HeLa cells showing reduced cytotoxicity against mammalian cells. (F) Structure of six dipeptide-based cationic amphiphiles 1–6 with C-14 aliphatic chains. (G) Scanning electron microscopic (SEM) images of (i) control bacterium E. coli; (ii) E. coli after treatment with 50 µgml−1 of amphiphile 1; (iii) control bacterium S. aureus; and (iv) S. aureus after treatment with 1.0 µg ml−1 of amphiphile 1 (scale bar = 0.5 µm) (figures 6(A) and (B) are adapted/modified from [77] with permission from American Chemical Society, copyright 2002; figures 6(D)–(E) from [79] with permission from John Wiley and Sons, copyright 2008; and figure 6(G) from [81] with permission from Royal Society of Chemistry, copyright 2009).
Download figure:
Standard image High-resolution imageMembrane-targeting AMPs are part of our body's innate immune system against microbial infections [78]. AMPs are facial amphiphiles with positively charged residues that bind with negatively-charged microbial membranes, and hydrophobic residues get incorporated into the hydrophobic lipid bilayer, disrupting the cell membrane of microbes. Authors developed L-Tryptophan-based five cationic amphiphiles having a positively charged quaternary ammonium group and varying alkyl chain length (figure 6(C)). All the amphiphiles could form a hydrogel in water, and as the alkyl chain is increased from 10 to 18 carbon chain length, the MGC (minimum gelation concentration) of the amphiphiles diminished from 15% to 0.2%. The antibacterial assay showed that MIC99 for these amphiphiles ranged from 0.1 to 1.0 µg ml−1 against Gram-positive bacteria. Amphiphiles 1–3 with C10, C12, C14 chains have MIC99 from 0.5 to 5.0 µg ml−1 for Gram-negative bacteria. In contrast, amphiphiles 4 and 5 with C16 and C18 carbon chains were less effective in killing Gram-positive bacteria, and ineffective in killing Gram-negative bacteria. Hemolytic activity (HC50) for amphiphiles ranged from 100 to 250 µg ml−1 for 1–3 amphiphiles, and was ~1000 µg ml−1 for amphiphile 4 and 5 (figure 6(D)). Cytotoxicity assay on HeLa cells showed <25% cell death at 500 µg ml−1 depicting the selectivity of cationic amphiphiles against negatively-charged bacterial membranes (figure 6(E)) [79].
Dipeptide-based amphiphiles where the C16 chain was tethered to different dipeptide scaffolds were synthesized by Mitra et al. Peptide head groups were fine-tuned to modulate the stability and aggregation properties of amphiphiles. Dipeptide-based amphiphiles can form a hydrogel in an aqueous solution, and the authors predicted that these hydrogels could be potent antimicrobial agents [80]. They further used the C14 alkyl chain on these dipeptides and evaluated their antimicrobial activities against Gram-positive and Gram-negative bacteria. Amphiphiles 1–5 (figure 6(F)) showed a very low MIC99 of 0.1–1.0 µg ml−1 against Gram-positive bacteria. In contrast, only amphiphile 1 showed better efficacy against Gram-negative and Gram-positive bacteria (MIC99 of 0.5–10 µg ml−1) that was confirmed by SEM images showing ruptured bacterial membranes after treatment (figure 6(G)). Amphiphile 1 was more effective against fungal species with MIC99 of 1–5 µg ml−1. As cationic amphiphiles face a significant challenge of biocompatibility against mammalian cells, the amphiphiles' toxicity was tested against HepG2, SiHa, and HeLa cells, and lower concentrations of amphiphiles were found to be non-toxic [81].
5.2. Drug encapsulated LMWHs for combating infectious diseases
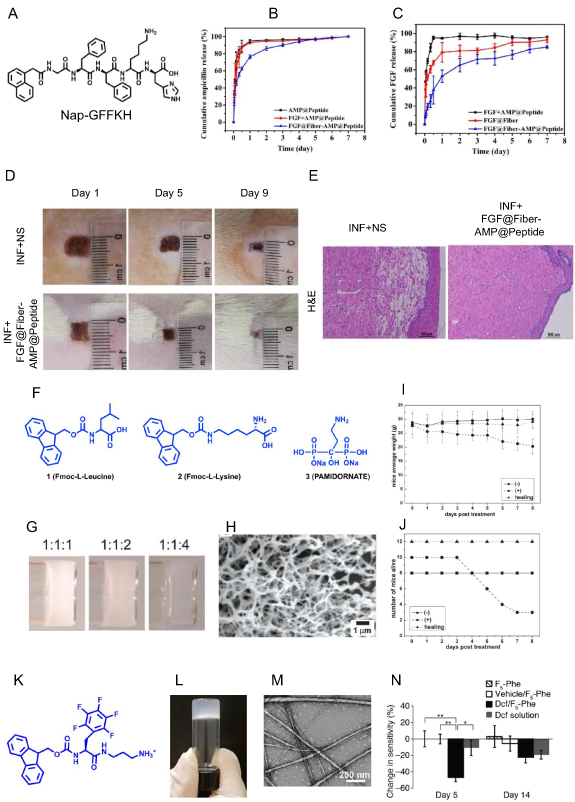
Skin is a protective organ that acts as a boundary for the outside environment and acts as a shield against external microorganisms [82]. Loss of skin integrity exposes the subcutaneous tissue to microbes for colonization and proliferation, and colonization of unattended wounds by pathogenic microbes increases the risk of infection. Once infected, the wound healing process is delayed, and the growing bacterial burden in the damaged tissue overcomes the host immune system, and it starts invasion and dissemination in surrounding tissues. Provoking the local host immune response leads to pus formation, pain, and erythema. Bacteria is the most common pathogen that causes skin infections [83], and S. aureus is mostly responsible for skin and soft tissue infection and is the leading cause of healthcare-associated diseases [84]. Hydrogels can enhance the entrapment efficacy of antibiotics and increase the bioavailability while maintaining the sustained release of antibiotics at the wound site. Zhong's lab developed a novel dual delivery system comprising of self-assembled peptide Nap-GFFKH that can encapsulate alginate microfibers at pH 6.0 to form hydrogel (figure 7(A)). The authors then used fibroblast growth factor (FGF) loaded alginate microfibers and encapsulated them in antibiotic (ampicillin, AMP) incorporated peptide to form a chimeric hydrogel FGF@Fiber-AMP@Peptide. They hypothesized that the delivery of antibiotics and growth factors would help mitigate the infection and fast wound healing. Drug release profile from FGF@Fiber-AMP@Peptide showed a burst release of AMP for combating infection with a sustained FGF release that can facilitate wound healing (figures 7(B) and (C)). Application of FGF@Fiber-AMP@Peptide showed mitigation of wound infection and significant wound healing in S. aureus infected SD rats (figure 7(D)). Minimal leukocyte infiltration and a large number of fibroblast proliferation was observed in H&E sections of FGF@Fiber-AMP@Peptide treated SD rats, thereby confirming its potential for wound healing (figure 7(E)) [85].
Figure 7. The drug encapsulated LMWHs for combating infectious diseases. (A) The molecular structure of Nap-GFFKH encapsulates alginate microfibers at pH 6.0 to form the hydrogel. (B), (C) Cumulative release of ampicillin (B) and FGF (C) from FGF@Fiber-AMP@Peptide hydrogel shows the sustained release of antibiotics and FGF. (D), (E) Pictorial representation and H&E stained micrographs of the wound on SD rats infected with S. aureus after applying FGF@Fiber-AMP@Peptide hydrogel confirm the clearance of infection and healing of wounds. (F) Molecular structure of Fmoc-leucine, Fmoc-lysine, and pamidronate. (G) Picture of hydrogel I, II, and III formed from different ratios of Fmoc-leucine, Fmoc-lysine, and pamidronate. (H) SEM structure of hydrogel III. (I), (J) Change in body weight change (I) and survival (J) of uranyl nitrate-mediated wounded mice on treatment with hydrogel III confirms wound healing. (K) Molecular structure of N-fluorenyl methoxycarbonyl phenylalanine (Fmoc-F5-Phe-DAP) derivative modified with diaminopropane at the carboxy-terminal. (L) Pictorial representation of Fmoc-F[5]-Phe-DAP hydrogel in an inverted vial. (M) SEM micrograph of Fmoc-F5-Phe-DAP hydrogel. (N) Change in mice paw sensitivity on days 5 and 14 post-treatment with diclofenac encapsulated Fmoc-F5[5]-Phe-DAP hydrogel confirm the reduction in pain (figures 7(A)–(E) are adapted/modified from [85] with permission from American Chemical Society, copyright 2020; figures 7(G)–(J) from [86] with permission from Royal Society of Chemistry, copyright 2008; and figures 7(L)–(N) from [87] with permission from American Chemical Society, copyright 2019).
Download figure:
Standard image High-resolution imageYang et al engineered a LMWH having Fmoc-L-leucine (1), Fmoc-L-lysine (2), and pamidronate (3) in three different molar ratios of 1:1:1; 1:1:2, and 1:1:4 (figure 7(F)), and found that mixture at a ratio of 1:1:1 and 1:1:2 formed an opaque hydrogel, whereas a ratio of 1:1:4 resulted in transparent hydrogel (hydrogel III) with higher storage modulus and dense nanofiber structure (figures 7(G) and (H)). The authors further tested hydrogel III's efficacy on simulated uranium wound formed by scratching the back of mice. Hydrogel III treated mice exhibit a slight weight loss with no mortality than control wounded mice that showed more than 35% weight loss with high mortality (figures 7(I) and (J)). The authors hypothesized that L-leucine and L-lysine migrate into the wound and hinders the infiltration of neutrophils while the pamidronate chelates with UO2 2+. Therefore, hydrogel III has the potential to be effective against wound healing and radioactive poisoning [86]. Aqueous solution of cationic N-fluorenyl methoxycarbonyl phenylalanine (Fmoc-Phe) derivative modified with diaminopropane at the carboxy-terminal allows it to self-assemble into hydrogel by addition of sodium chloride (figures 7(K) and (L)). Fmoc-F5-Phe-DAP showed high gel strength, and SEM images showed twisted fiber and tape-like structure (figure 7(M)). The Fmoc-F5-Phe-DAP hydrogel was shown to encapsulate diclofenac, a nonsteroidal anti-inflammatory drug (NSAID), that showed slower release than other hydrogels of Fmoc-Phe-DAP. Authors developed an induced pain model in mice using an intra-articular injection of complete Freund's adjuvant (CFA) into a hind limb ankle joint, and administration of diclofenac encapsulated Fmoc-F5-Phe-DAP hydrogel to the distressed ankle joint remediated the pain (figure 7(N)) [87].
The biocompatible and non-immunogenic nature of LMWHs makes them an ideal delivery system than the excipient used in cream or ointment formulations of antibiotics, which is known to cause local toxicity and irritation. The conjugation of active molecules to scaffold forming hydrogel enhances the solubility and bioavailability of antibiotics. The conjugation also prevents the drug's burst release from hydrogels and extends the antibiotics' antibacterial activity. The hydrogels formed from conjugates containing charged head groups help in adherence of molecules on the bacterial surface and disrupt the membrane with decreased resistance chances, thereby characterizing them as excellent antimicrobial agents for topical drug delivery. The LMWHs are employed to entrap antibiotics with high entrapment efficacy, and the sustained release of the entrapped antibiotic has accorded these antibiotic-loaded hydrogels a cut above marketed formulations. The entrapped and conjugated antibiotics in the LMHWs have been shown to prolong the life and the antibiotic activity that aids in the effective treatment of infections. Hence the LMWHs formed by intermolecular non-covalent interactions are useful soft biomaterials for topical drug delivery applications.
6. Hydrogels for inflammatory disorders
Inflammation is a biological response to an immunogen by the host defense system. Rheumatoid arthritis, Atopic dermatitis, Flares, Gout, Psoriasis, Colitis, and Crohn's disease are results of chronic inflammation. Chemotherapy for inflammatory diseases needs management of inflammation by glucocorticoids, NSAIDs, disease-modifying antirheumatic drugs (DMARD), and biological agents. NSAIDs help in relieving pain, swelling, fever, inflammation, and other symptoms of the disease. Aspirin, a cyclooxygenase inhibitor, is the first anti-inflammatory drug synthesized by Hoffman [88]. Glucocorticoids like dexamethasone, betamethasone, and prednisone effectively control and treat inflammatory and autoimmune diseases. They reduce the expression of cytokine-induced genes and increase the expression of anti-inflammatory cytokines. Long-term use of glucocorticoids induces severe side effects, including osteoporosis, dyslipidemia, hypertension, and diabetes mellitus [89]. DMARDs, including methotrexate, cyclosporine, tacrolimus, cyclophosphamide, rapamycin, and immunosuppressive and immunomodulatory agents, are used for rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis, but their use is associated with severe side effects [90]. Long term systemic delivery of glucocorticoids and DMARD's causes unwanted and severe side-effects. Therefore, there is a need for an adequate drug delivery vehicle that can minimize the side-effects and improve the efficacy of these anti-inflammatory drugs. LMWH-based delivery of anti-inflammatory drugs at the target site can increase the local tissue concentration, and therefore can limit the medication's systemic circulation and prevents the long-term side effects on the human body.
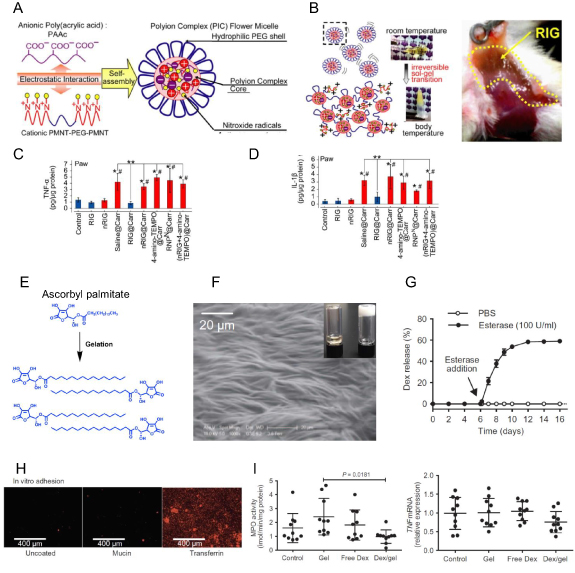
Nitroxide-radicle compounds (TEMPO) are well known catalytic ROS scavengers that help in combating inflammation. Nitroxide-radical containing NP (RNPN) was prepared by dissolving cationic PMNT-PEG-PMNT (poly[4-(2,2,6,6-tetramethyl piperidine-N-oxyl) aminomethylstyrene]) triblock copolymer and anionic PAA (polyacrylic acid) in disodium hydrogen phosphate buffer (figure 8(A)). This RNPN, termed as a redox-active injectable gel (RIG) precursor, is 48–89 nm in size. Its zeta potential changes from negative to positive upon an increase in the molar ratio of PAA from 4:1–1:2. RIG precursor solution in 1:1 (RIG: PAA) molar ratio forms the gel at 37 °C in vitro and upon subcutaneous injection in mice (figure 8(B)). Authors further showed that RIG pre-treated mice were able to decrease the effect of localized inflammation induced by carrageenan injection into the footpad of mice that was confirmed by quantification of proinflammatory cytokines TNF-α and IL-1β (figures 8(C) and (D)) [91].
Figure 8. (A) Schema of redox-active injectable hydrogel (RIG) system. (B) Sol to gel transition of RIG at body temperature in vitro and in vivo gelation after subcutaneous injection of 100 μl of flower micelle solution into the mouse thigh. (C), (D) The decrease in the expression of proinflammatory TNF-α (C) and IL-1β (D) in mice hind paw upon carrageenan-induced arthritis confirms the protective effect of RIG hydrogel. (E) Structure of ascorbyl palmitate (AP) and its self-assembly to form a gel. (F) SEM image of inflammation targeting hydrogel (IT-Gel) showing microfibrillar structure (insert: an optical image of IT-hydrogel). (G) Drug release kinetics studies showed the esterase-mediated release of DEX from IT-hydrogel. (H) Fluorescence micrographs showing the adhesion of IT-hydrogel on the simulated inflamed epithelium. (I) Change in MPO activity and TNF-α expression in the inflamed colon of mice showed a significant decrease in treatment with IT-hydrogel (figures 8(A)–(D) are adapted/modified from [91] and figures 8(F)–(I) from [93] with permission from American Association for the Advancement of Science, copyright 2013 and 2015).
Download figure:
Standard image High-resolution imageInflammatory bowel disease is a chronic inflammation of the GIT. Ascorbyl palmitate (AP) can act as a self-assembling amphiphile (figure 8(E)). It can form inflammation targeting hydrogel (IT-Gel) in DMSO/water solvent mixture upon heating and cooling at RT with microfibrillar structures (figure 8(F)). IT hydrogel successfully encapsulated the hydrophobic anti-inflammatory drug (dexamethasone) and maintained its sustained release in the presence of esterase (figure 8(G)). Authors hypothesized that IT hydrogel's negatively-charged surface could bind to positively charged inflamed colonic mucosa devoid of the mucus layer (figure 8(H)) [92]. The colitis TRUC mice receiving IT hydrogel showed a significant reduction in the disease severity as seen by H&E staining and decreased MPO activity and TNF expression (figure 8(I)) in the distal colon compared to control mice [93].
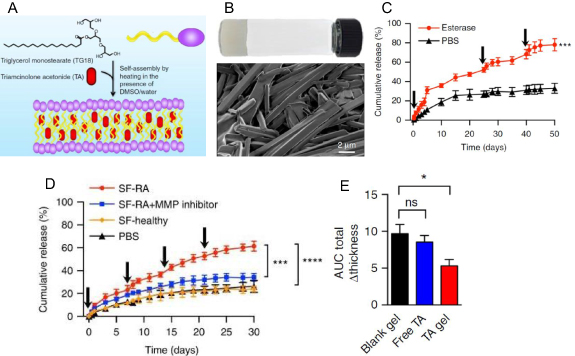
Arthritis is an inflammatory disorder causing swelling and tenderness of joints accompanied by pain, stiffness, redness, and decreased motion of joints. Triglycerol monostearate (TG-18) can self-assemble into fibrous structure hydrogel in DMSO/water mixture upon heating to 60 °C and cooling to RT. TG-18 hydrogel can encapsulate hydrophobic drug like triamcinolone acetonide (TA) as confirmed by high-resolution SEM studies (figures 9(A) and (B)), and can release the cargo in the presence of esterase, MMP-2, 3, and 9 (figure 9(C)). Synovial fluid from rheumatoid arthritis patients caused enhanced drug release kinetics from TA-loaded TG-18 hydrogel (figure 9(D)). Authors then used an inflamed-arthritis (IA) model induced by intraperitoneal injection of K/BxN serum into C57BL/6 mice and showed that TA-loaded TG-18 hydrogel injected in the right hind paw reduced the arthritis severity and swelling (figure 9(E)) [94].
Figure 9. (A) Schema showing the triglycerol monostearate amphiphile and its ability to self-assemble and encapsulate triamcinolone acetonide (TA) to form the hydrogel. (B) Optical and high-resolution SEM image of TA-loaded TG-18 hydrogel. (C), D) Cumulative release of TA from TA-loaded TG-18 hydrogel in the presence of esterase (C) and synovial fluid (D) shows the esterase-mediated release of the TA. The fresh enzyme was added at the indicated time points shown by arrows. (E) Quantification of the change in total paw thickness using induced arthritis model by injection of K/BxN serum shows a significant decrease on treatment with TA-loaded TG-18 hydrogel (TA gel) (figures 9(A)–(E) are adapted/modified from [94] with permission from Nature, copyright 2018).
Download figure:
Standard image High-resolution imageAnti-inflammatory drugs are critical for vascularized composite allotransplantation (VCA) like neck, scalp, skull, and uterus transplantation. Lifelong use of immunosuppressant causes nephrotoxicity, hepatotoxicity, diabetes mellitus, leukopenia, hypertension, opportunistic infections, and deteriorates life quality [95]. Tacrolimus (TAC), a calcineurin inhibitor, is commonly used as maintenance therapy in VCA (figure 10(A)), but its systemic delivery is associated with nephrotoxicity and diabetogenicity. Triglycerol monostearate (figure 10(B)) is known to self-assemble into the hydrogel that can disassemble in the presence of esterases and matrix metalloproteases (MMP) overexpressed at the inflammation site. TGMS can encapsulate TAC and form stable hydrogel at physiological temperature with nanofiber structures. The drug release profile of TAC from TGMS–TAC under lipase, MMP-2, and MMP-9 showed >90% release within 21 d, and less than 10% in PBS in 28 d (figure 10(C)). Supernatant from LPS-activated macrophages also enhanced the drug release from TGMS-TAC hydrogel. A single subcutaneous injection of TGMS-TAC hydrogel in hind limb transplanted from Norway-to-Lewis rat increased the median survival of graft to >100 d compared to no treatment with a median survival of 11 d (figure 10(D)). Minimal mononuclear cell infiltration was seen by H&E of skin and calf muscles in TGMS-TAC treated graft (figure 10(E)). There was also a significant decrease in levels of cytokines IL-2, TNF-α, IFN-γ, and IL-1β responsible for anti-graft immune response in the TGMS–TAC hydrogel injected group compared to untreated control (figure 10(F)). Therefore, this study establishes the use of TGMS–TAC hydrogel for immunosuppressive drug delivery in the animal transplantation model [96].
Figure 10. (A), (B) Structure of tacrolimus (A) and triglycerol monostearate (B) used in TGMS–TAC hydrogel. (C) The drug release profile of tacrolimus (TAC) from TGMS-TAC hydrogel in the presence of lipase, MMP-2, and MMP-9 confirms the gel's stimuli-responsive nature. (D) The survival graph of transplanted graft mice shows >100 d increase in survival of mice on treatment with TGMS–TAC hydrogel. (E) The H&E micrograph of skin sections of graft tissue after the treatment with TGMS–TAC hydrogel shows decreased severe cell infiltrations, edema formation, and necrosis compared to no treatment skin section. (F) Quantification of cytokines IL-2, TNF-α, IFN-γ, and IL-1β from mice plasma receiving grafts and treatment with TGMS–TAC hydrogel showed decreased levels post-operation day 7 confirm that TGMS–TAC gel is effective in inhibiting anti-graft immune response (figures 10(A)–(D) are adapted/modified from [96] with permission from American Association for the Advancement of Science, copyright 2014).
Download figure:
Standard image High-resolution imageLMWHs can improve the delivery of anti-inflammatory drugs to the target site by exploiting the inflamed tissue for adhesion and release of the entrapped drug (anti-inflammatory) at the target site. Electrostatic interactions between hydrogels and negatively charged surface of the inflamed tissue prolonged drug release from the hydrogel and result in effective treatment. LMWHs containing enzyme sensitive linkers get triggered by the abundance of esterases and MMP at the inflammation site to release the active molecule at the target site. Localized, sustained, and prolonged delivery of immunosuppressants entrapped in hydrogels at allograft sites has been shown to play a significant role in preventing graft rejections and harmful side effects of glucocorticoids. Overall, hydrogels have improved the treatment of inflammatory disorders with targeted delivery and prolonged release of the drug at the inflammation site.
7. Conclusions and future directions
Oral administration, the safest and most convenient drug delivery option, is limited by decreased bioavailability, short half-life in circulation, and poor targeting. In contrast, intravenous injection increases the drugs' bioavailability but is limited by rapid renal clearance, increased systemic toxicity, and poor targeting. Challenges of conventional chemotherapy have been addressed by nanotechnology to some extent by controlled delivery and reduced toxicity of drugs where NPs, liposomes, polymer-based NPs, and hydrogels have emerged as better options against conventional chemotherapy. These DDSs have revolutionized the way chemotherapeutic drugs are delivered by controlling their release kinetics over time and in space, enhancing their efficacy, reducing their toxicity and required dosage. Hydrogel-based DDS has come a long way that help to overcome conventional drug delivery challenges, and especially the LMWHs have emerged as indispensable scaffolds for drug delivery over polymeric hydrogels. The non-covalent interactions in supramolecular self-assembly of amphiphiles in hydrogels make these hydrogels biodegradable than chemical cross-linking in polymer gels. Integration of stimuli like temperature, pH, and enzyme responsible chemical moieties can further allow the fine-tuning of drug release kinetics. There are still several challenges to advance the clinical applicability of LMWHs as drug delivery vehicles for chemotherapeutics.
As many chemotherapeutic treatments need a combination of drugs, delivery of multiple drugs from LMWHs maintaining their sequential and controlled release in the desired manner is challenging. Few LMWHs have established the entrapment and release of a combination of drugs, but ascertaining each drug's release at a different rate remains a challenge. The entrapment of the combination of hydrophobic and hydrophilic drugs can further hinder the entrapment efficacy as it disrupts the supramolecular interactions. Therefore, engineering of LMWHs having the combination of drugs with the on-demand release of specific molecules targeting different pathways could enhance the therapeutic efficacy of drugs in cancer and infectious disease like tuberculosis. Another challenge of LMWH is to prolong the duration of the release of drugs. An ideal hydrogel-based system can be fine-tuned to release the drug in the required amount depending on the increase or decrease of demand over time for long-term release. Stimuli-responsive hydrogels with different degradation profiles may address these issues. The delivery of biological molecules like proteins, antibodies, and nucleic acids, such as RNA and DNA that can be easily unfolded, denatured, or deactivated by the hydrogel, is another big challenge. Biological activities of these entrapped biomolecules are readily affected by the hydrophobic domains and the functional groups in the thermal and physically gelling hydrogels. The development of hydrogels capable of entrapping such biological agents without affecting their structure and activity could significantly enhance the therapeutic efficacy. The most common challenge for most biomaterials used for biomedical applications is their biocompatibility, and hydrogels are no exception. LMWHs with better biocompatibility can find their use in the implants and drug depots inside the human body as carriers of chemotherapy for different diseases like cancer, wound infections, diabetes, and graft transplantation. Any biomaterial injected or transplanted into the human body tends to evoke an immune response. Therefore, the biomaterial is biocompatible only if there is a minimal infiltration of leukocytes and macrophages at the injection or implantation site. Immune infiltration results in inflammation and may lead to early disintegration of molecular hydrogels. An extensive study on biocompatibility and biodegradability of hydrogels is essential. In summary, addressing the above challenges can make the LMWHs potential candidates for clinical applications.
Acknowledgments
This work in our lab is supported by RCB, Department of Biotechnology, Department of Science and Technology, and Science and Engineering Research Board, India. We thank DBT e-Library Consortium (DeLCON). Some figures were created with BioRender.com.