Abstract
Advanced biomaterials are increasingly used for numerous medical applications from the delivery of cancer-targeted therapeutics to the treatment of cardiovascular diseases. The issues of foreign body reactions induced by biomaterials must be controlled for preventing treatment failure. Therefore, it is important to assess the biocompatibility and cytotoxicity of biomaterials on cell culture systems before proceeding to in vivo studies in animal models and subsequent clinical trials. Direct use of biomaterials on animals create technical challenges and ethical issues and therefore, the use of non-animal models such as stem cell cultures could be useful for determination of their safety. However, failure to recapitulate the complex in vivo microenvironment have largely restricted stem cell cultures for testing the cytotoxicity of biomaterials. Nevertheless, properties of stem cells such as their self-renewal and ability to differentiate into various cell lineages make them an ideal candidate for in vitro screening studies. Furthermore, the application of stem cells in biomaterials screening studies may overcome the challenges associated with the inability to develop a complex heterogeneous tissue using primary cells. Currently, embryonic stem cells, adult stem cells, and induced pluripotent stem cells are being used as in vitro preliminary biomaterials testing models with demonstrated advantages over mature primary cell or cell line based in vitro models. This review discusses the status and future directions of in vitro stem cell-based cultures and their derivatives such as spheroids and organoids for the screening of their safety before their application to animal models and human in translational research.
Export citation and abstract BibTeX RIS
1. Introduction
Advances in tissue culture techniques have recently revealed that testing the biomaterials on cell lines such as stem cells play an important role in the early determination of their biocompatibility, cytotoxicity, and genotoxicity of biomaterials. Biomaterials cytotoxicity assessment usually starts with the development of monolayer cell culture systems and later proceeds with animal models and ends with clinical studies in human volunteers. Biocompatibility and cytotoxicity evaluation are preliminary biomaterials screening tests carried out to ensure that these materials selected for use in devices are safe for their intended use [1]. The screening of biomaterials is performed either in vitro using appropriate cells that play an important role in biomedical applications, or in vivo via implantation or injection of the target test material [2]. Biomaterials that show good biocompatibility and cytotoxicity during in vitro trials also exhibit similar properties in vivo experiments which exhibit the usefulness of in vitro cultures as systems to be select the biomaterials for diverse biomedical applications [3]. Screening the biological properties of biomaterials in vitro trials is easier and robust while relevant in vivo tests are quite laborious and time taking [4]. Sometimes, biomaterials may be extremely toxic to specific tissues, it is necessary to check their biocompatibility by testing their cytotoxic effects on cell cultures before their testing in animals and on the human body [5]. Direct use of biomaterials on animals may create serious ethical issues including pain and distress [6]. Cytotoxicity of newly developed biomaterials is essential to be assessed on animals because animal models share relatively comparable molecular pathways with that of human beings [7–9]. However, intensive use of animals for cytotoxicity evaluation of biomaterials on millions of animals like mice, rabbits, dogs, and monkeys could lead create a huge financial burden [10–13]. In addition, direct use of biomaterials for cytotoxicity studies on animals may cause skin irritation, acute cytotoxicity, and reproductive cytotoxicity [14, 15]. Moreover, biomaterials safety assessed in animals' models does not mean that they will show the same dose-response while testing their effectiveness in humans. Studies show that about 40% of materials are withdrawn during clinical-trials due to their cytotoxicity not detected up-stream. Keeping in view the ethical issues and high cost of the use of animals in biomaterials screening evaluations, 3Rs (replacement, reduction, and refinement) concept was introduced in 1959 to minimize the use of animals in different studies such as cytotoxicity assessment of biomaterials [16, 17].
In vitro cell cultures such as stem cell models are of great value because they can provide rapid response to the biomaterials, relatively easy to maintain, and are non-laborious as compared to in vivo models [18, 19]. In addition, in vitro based methods use well-developed cell culture protocols that can be performed at controlled conditions and thus can provide accurate and reproducible results as compared to in vivo models [20, 21]. There are different kinds of stem cells that have a wide scale of applicability in biocompatibility and cytotoxicity studies such as embryonic stem cells (ESCs) [22], mesenchymal stem cells (MSCs) [23–26], and human-induced pluripotent stem cells (hiPSCs) [27–29], displaying either pluripotent or multipotent properties which help them to differentiate into various cell lineages [30–32]. For instance, pluripotent stem cells such as beating cardiomyocytes and functional hepatocytes have been successfully derived from ESCs which have wide-scale applicability in biomaterials screening studies [33–36]. In short, stem cell-based systems and their ability to provide a heterogeneous combination of cells for biomedical material testing, the chances of passing through animal testing trials and clinical trials have been tremendously enhanced for the development of better therapeutic regimens [37].
The use of stem cells for biomaterials cytotoxicity evaluation is not a straightforward process as several challenges are encountered during preliminary screening assays. For example, culture conditions, phenotypic characterization, and selection of cells, duration of experiments, dosing regimen, and selection of potential biological endpoints in the context of human risk may largely affect in vitro assays involving stem cells [38]. Besides the use of stem cells such as induced pluripotent stem cells (iPSCs) had its inherent difficulties because these cells are produced by the reprogramming of somatic cells and may easily lose their plasticity and desired phenotype during the reprogramming process.
Several review articles before this have been published that highlight the use of cell cultures for drug discovery and development [39–43]. This review will instead discuss stem cell models for studying biocompatibility and cytotoxicity of biomaterials for assessment of their safety before proceeding to the testing them on animals and clinical trials on humans. The review also presents extensive studies on the three-dimensional (3D) stem culture models such as spheroids and organoids which offer a similar microenvironment as present in the animal tissues. This review presents the studies related to stem cell as disease models of different diseases for screening the cytotoxic effects of biomaterials mostly published in last 5 years. The literature cited here was found via PubMed (NCBI) and google scholar using keywords like stem cell models, biomaterials cytotoxicity, non-animal methods, ESCs, organ on a chip model. Moreover, articles on all types of stem cells were cited under consideration including adult stem cells (ASCs), iPSCs, and ESCs during the literature survey. This review contains immense literature and is hoped to explore the potential of different types of stem cells like MSCs, iPSCs, and ESCs in the assessment of biomedical material screening studies using in vitro cultures.
2. Assays for biomaterials cytotoxicity assessment
Over the last few decades, novel assays have been developed in the form of non-animal methods for the safe screening of biomaterials which have subsequently eliminated the painful procedures needed for in vivo studies and in this way, the welfare of animals has been prioritized [44]. Multiple types of assays have been developed like hen's egg test-chorioallantoic membrane for the screening of the cytotoxic effects of drugs that are associated with the irritation of the eye [45]. Chicken embryo is another type of cytotoxicity testing for evaluating the effects of various biomaterials on the angiogenic activity [46–49] and gene expression [50]. The cytotoxicity of biomaterials on cornea is evaluated by Bovine Cornea Opacity/Permeability assay that is performed by extraction of by-products from slaughtered cows [51, 52]. Eyes from dead rabbits and chicken had been also used to evaluate the biomaterials irritation effects on the eyes [53, 54]. Scientists have developed 3D epithelial models using human skin-derived epidermal keratinocytes and human corneal epithelial cells (HCE), which are available commercially having a trademark name EpiOcular™ and SkinEthic™ respectively [55, 56]. Neural red uptake assay is used to assess human keratinocyte viability [57] while, Corrositex™ assay is for determining the biomaterials corrosive effects on skin, therefore, avoiding the need of use of animal models [58]. Another form of cytotoxicity assessment model is 'skin-on-a-chip' platform which is capable to measure skin inflammation and skin cell viability [59].
Some of materials may produce adverse effects on reproduction (teratogenicity) and may lead to DNA damage and therefore, Caenorhabditis elegans is a suitable model for screening cytotoxicity studies [60]. Additionally, Zebrafish and its embryo has been extensively used for studying effects of materials to the ocular, cardiovascular and neural differentiation [61–64]. Some of the in vitro models used in cytotoxicity studies of biomaterials are shown in table 1.
Table 1. In vitro cytotoxicity study models.
| Serial no. | Name of assay | Condition of toxicity analysis | References |
|---|---|---|---|
| 1 | Hen's egg test-chorioallantoic membrane (HET-CAM) | Eye irritation | [65] |
| 2 | Bovine Cornea Opacity/Permeability (BCOP) | Cytotoxicity of cornea | [51, 52] |
| 3 | EpiOcular™ | 3D epithelial models using human skin-derived epidermal keratinocytes | [55] |
| 4 | SkinEthic™ | 3D epithelial models using human corneal epithelial cells (HCE) | [56] |
| 5 | Neural red uptake (NRU) | Assessment of human keratinocyte viability | [57] |
| 6 | Corrositex™ | Skin corrosive potential validation | [58] |
| 7 | Skin-on-a-chip | Skin inflammation and skin cell viability testing | [59] |
| 8 | Caenorhabditis elegans | Cytotoxicity effects on reproduction and DNA damage done by drug | [60] |
| 9 | Zebrafish embryo | Cardiovascular, neural, and developmental cytotoxicity studies | [61–63] |
3. Stem cells models for cytotoxicity studies
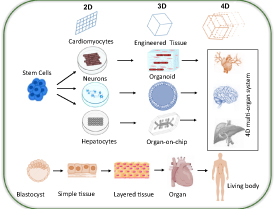
Modern biological and biomedical science is being powered by model systems that involve the use of stem cells. The purpose of these model systems is to recapitulate the functions and processes of the body from the molecular level to the level of the cell, tissue, organ, or whole organism. The body can be interpreted as a total of a vast number and wide range of highly ordered cellular and non-cellular materials (e.g. cell, tissue, and organ) as well as the whole interactome that involves interactions inside (e.g. cell–cell, cell–matrix) or external (e.g. cell-environment) [66]. Stem cells in cytotoxicity studies are used for biomaterials screening applications and generating comprehensive biomaterials cytotoxicity profiles [67]. In vitro profiling of cytotoxicity effects of food additives, pharmaceutical, and medical devices on stem cells reveals their potential hazards before proceeding to the trials on animals and humans [68]. Historically, cytotoxicity profiling of biomaterials has entirely relied on animals. But over the few decades, the use of stem cells for biomaterials biocompatibility and cytotoxicity profiling is immensely promising in translational research as this has largely reduced the high costs of use of animals but also produce sophistical cytotoxicity profiling results besides cheaper and faster compared to animal-based cytotoxicity testing [69]. The testing of cytotoxicity of materials on stem cell cultures is carried out under the guidelines of different agencies. For instance, the guidelines for testing the materials are included in ISO 10993 and ISO 5832-1 series and cytotoxicity evaluation is recommended to be carried out under ISO 10993-5 guidelines which clarify extraction testing method, indirect contact method and the direct contact method for evaluation of in vitro cytotoxicity studies [70–72]. Alternative guidelines for use of materials for cytotoxicity studies are presented in ASTM F136 and ASTM F139 series. The basic aim of these guidelines is to use those materials that are non-toxic and have shown a long track of being safe in drug testing trials [73, 74]. It is most important to follow such guidelines and standards when stem cell-based approaches are used in biomedical material cytotoxicity testing. The ability to differentiate into multiple lineages and self-renewal [28, 75], enables stem cells to be used for biomaterials cytotoxicity analysis. Because stem cells can be differentiated into a vast variety of mature cells such as cardiac, adipose, liver, neurons, fibroblasts, muscle, skin, and blood cells [76]. Thus, enabling them to be used in almost any type of study alternative to animal models. They give rise to differentiated cells that are the basic units of tissues and organs. The self-renewal property, and differentiation capacity to form different cell types from a single cell are the unique features of stem cells [77]. The advantage of using stem cells derived from specific tissues is that stem cells could be differentiated into tissue-specific mature cells. The use of differentiated stem cells or organoids as a disease, model thus, will reflect the 3D microenvironments present in tissues and thus will mimic the histology and physiology of animal models as in vivo studies (figure 1) [43, 78].
Figure 1. Shows different types of stem cell systems (2D, 3D, and 4D) and their potential to differentiate into different types of models which are currently used in biocompatibility and cytotoxicity studies of biomaterials.
Download figure:
Standard image High-resolution imageStem cells can be categorized mainly into three types: (a) ESCs, (b) ASCs, and (c) iPSCs. The focus of stem cell research is to use them as regenerative medicine, but studies suggest they can be used for cytotoxicity studies. Using human stem cells can help in better understanding of toxic effects of biomedical material and drugs on human tissues thus making them perfect for testing biomaterial and drugs rather than animal models [79]. Another important advantage is that stem cells can self-renew themselves almost unlimitedly and can differentiate into many cells lineages [80] and can be used to form spheroids, organoids and organ-on-chips for which can mimic the exact properties of a specific organ.
The popularity of two-dimensional (2D) micropatterns and 3D stem cell culture systems has seen a boom in the last 10 years to replicate the architecture and interactions of specific cell populations during growth. These techniques, using cell lines instead of embryos, allow the regulated exploration of cell organization and patterning during development. For preimplantation and peri-implantation embryogenesis, a new class of in vitro model systems has been developed, ranging from blastocyst stage models through gastrulation and early organogenesis [81]. A newly evolved tissue engineering technique is to biofabricate tissues as building blocks using cellular spheroids. Massive amounts of stem cells, with a diameter of hundreds of micrometers, are first collected and converted into multicellular spheroids by techniques like re-aggregation, microfluidics, hanging drop, micro-molds, spinner culture, and rotating wall vessels [82–85]. Cells are centrifuged in a tube using the re-aggregation process to create a pellet that is then sliced into tiny pieces that are eventually cultured to form spheroids [86]. It is easy to biofabricate tissues by first fusing human pluripotent stem cell (hPSC) spheroids, followed by separation and maturation.
Organoids have been developed by mimicking the biochemical and physical signals of tissue growth and homeostasis from both pluripotent stem cells (PSCs) and ASCs [87]. Differentiated cells from PSCs can self-organize to form tissue-specific organoids like the optic cup, brain, intestine, liver, and kidney if the appropriate 3D scaffold and biochemical conditions are given to them [88–92]. In order to regulate stem cell fate, existing organoid systems depend mainly on intrinsic or extrinsic biochemical signals (e.g. growth factors) and cell-autonomous or cell–cell interactions. Although all of these are important factors for regulating cell differentiation and organisation, organoid development is highly dependent on cell-autonomous self-organization, which is not easily regulated yet [87]. The effectiveness of spheroids and tissue explants can be due to their ability to replicate the complex interactions of niches present in situ. Organoids offer a more sophisticated in vitro instrument that allows for the execution of more physiologically important tests that cannot be done in animals or humans. With the vast array of bioengineering tools currently available, the effectiveness of organoids can be expanded with better control over external signals and with an unparalleled ability to track and exploit cellular conduct [66].
The integration of microfabrication and tissue engineering has given rise to organ-on-a-chip innovations, offering an alternative to traditional drug screening preclinical models. Organ-on-a-chip systems can mimic crucial elements of human physiology that are important for understanding the effects of medications, enhancing preclinical safety and testing for effectiveness [93]. Organ-on-a-chip technology incorporates certain factors to replicate main characteristics within a microfabricated system with unique tissue microenvironments and architectures, enabling the development of 3D models that display native tissue functional hallmarks [94]. Many organ-on-a-chip models including heart, nerve, liver have been constructed by scientists to simulate the biomedical material cytotoxicity effect on certain organs [94]. These organ-on-a-chip models have shown great potential in the development of better in vitro cytotoxicity detection platforms for the screening of biomaterials [95].
3.1. Hepatotoxicity studies
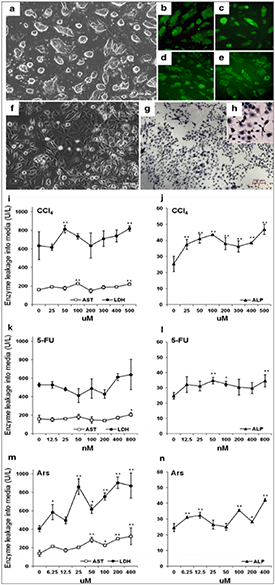
The liver is the major organ in the human body where detoxification of many compounds takes place thus biomaterials exhibit more adverse effects than any other organ [37]. To study in vitro cytotoxic effects of biomaterials, human primary hepatocytes are generally used as these cells can de-differentiate almost instantly on their isolation from the liver and continues to do so even after a week [96]. This opens opportunities for studying the biocompatibility and cytotoxicity of biomaterials on stem cells as these cells offer a good opportunity for the evaluation of cytotoxicity studies of the liver cells. For example, in one of the studies, ESCs were obtained after differentiation of hepatic cells and used as a potential candidate for screening the cytotoxic effects on the liver (figures 2(a)–(h)) [97]. In the context of in vitro testing of toxic agents, the treatment of carbon tetrachloride (CCl4), arsanilic acid (Ars), and 5-fluorouracil (5-FU) to the hepatic progenitor cells derived from ESCs leads to an increase in the activity levels of lactate dehydrogenase (LDH), aspartate transaminase (AST), and alkaline phosphatase (ALP) showing the potential of the use of ESCs for hepato-cytotoxicity studies (figures 2(i)–(n)) [97]. In another interesting study, hESC-derived hepatocytes were used to examine the drug metabolism by conversion of acetaminophen to N-acetyl-p-benzoquinone imine which is normally cytotoxic to cells [98]. Szkolnicka et al observed that hESC-derived hepatocytes showed more drug inducible activity as compared to iPSCs [99]. Lee et al used hepatocytes derived placenta-derived stem cells (PDSCs) to test the hepatotoxic effects of compounds like CCl4. The result showed that cell viability of PDSCs were higher compared to bone marrow-derived MSCs (BM-MSCs) during the treatment with CCl4 [100]. In another study, hiPSC-derived hepatocytes were used by Grimm et al to determine the safe levels of treatment of biomaterials on liver cells which is considered very important for new biomaterials discovery and drug development [101, 102]. In one study, iPSCs derived from α1antitrypsin (A1AT) deficient patients were isolated and applied to develop a compound that reduced defective (A1AT) present in the cytoplasm [103, 104]. Lu et al confirmed that the hiPSC-derived hepatocytes have human hepatocyte-like characteristics like specific protein expression and morphology and can be applied effectively as for the study of hepatotoxic studies [105].
Figure 2. Morphological features of hepatocyte-like cells (HCs) during differentiation from ESCs. ESCs exhibited spherical shape (a) and identified by Nanog (b), Oct4 (c), Sox2 (d), and SSEA-1 (e) expression. After differentiation, ESC was turned into to HCs showing large nucleus and dark granular deposit the (f). Periodic acid–Schiff (PAS) staining without magnification (g) and with magnification (h). The influence of CCl4 (i) and (j), 5-FU (k) and (l) and Ars (m) and (n) for 40 d on the activities of AST, LDH, and ALP in HCs. Reprinted with permission from [97].
Download figure:
Standard image High-resolution imageIn a study, hepatocyte growth factor (HGF) was introduced by ionotropic gelation into chitosan nanoparticles (CNP). The average size of the prepared nanoparticles was 100 nm, suggesting a stable release of HGF. The cytotoxicity research did not show any adverse effects of up to 4 mg CNP ml−1 culture medium on bone marrow MSCs. MSC was incubated with HGF-CNP and other supplements to determine the impact of HGF added CNP (HGF-CNP) on in vitro hepatic differentiation. The fibroblast-like morphology of MSC became round-shaped after 21 d, a typical trait of hepatocyte cells. Hepatic characterization was confirmed by an immunofluorescence analysis for albumin expression [107]. In another study, after 70% of hepatectomy, alginate scaffold-bone marrow stem cell (BMSC) complex (the experimental group) and alginate scaffolds (the control group) were placed on the wound site of rat liver. Albumin and glycogen were secreted by BMSCs on alginate scaffolds. The rate of survival and liver function of rats in the study group was substantially greater than that of rats in the control group. Alginate scaffold-complex BMSC facilitated liver tissue regeneration in rats with acute liver failure. This study concluded the biocompatibility of alginate-BMSCs scaffolds in the case of acute liver damage [108]. The further details of biomaterials cytotoxicity studies can be read from table 2.
Table 2. Use of various types of stem cells for screening cytotoxicity of different biomaterials and drugs.
| Type of stem cells | Species | Source of stem cells | Application | References |
|---|---|---|---|---|
| ESCs | Mouse | mESC derived hepatic progenitor cells | Chemical toxicity testing | [97] |
| Human | hESC derived hepatocytes | Drugs metabolism testing | [98] | |
| Human | iPSCs and hESC derived hepatocytes | Assessment of drugs inducible activity | [99] | |
| ASCs | Human | Hepatocytes derived placenta-derived stem cells (PDSCs) | Chemical cytotoxicity testing | [100] |
| Murine | BM-MSCs | Cytotoxicity of chitosan nanoparticles (CNP) | [107] | |
| Rat | BMSC | Biocompatibility of alginate scaffold | [108] | |
| iPSCs | Human | hiPSC derived hepatocytes | Safety testing of drugs | [101] |
| Human | hiPSC derived hepatocytes | Comparison of characteristics of hiPSCs derived hepatocytes with hepatocytes | [105] | |
| ESCs | Human | hESCs derived cardiomyocytes | Cardiotoxicity analysis | [109] |
| Human | hESCs derived cardiomyocytes | Cytotoxicity of doxorubicin | [110] | |
| ASCs | Human | MSCs | Biocompatibility of alginate microspheres with human mesenchymal stem cells (hMSCs) | [111] |
| Humans | ADSCs | Poly(lactide-co-glycolide)-monomethoxy-poly-(polyethylene glycol) nanoparticles cytotoxicity | [112] | |
| iPSCs | Human | Human iPSCs derived cardiomyocytes | Assessment of cell viability in the presence of doxorubicin | [113] |
| Human | hESC-derived cardiomyocytes and hiPSC-derived cardiomyocytes | Biomaterials and drugs cellular response assessment | [114] | |
| Mouse, human | mESC-derived cardiomyocytes and hiPSC-derived cardiomyocytes | Drugs cardiotoxic effects assessment | [115] | |
| Human | hiPSC derived cardiomyocytes | Cytotoxicity testing of matrine, sophocarpine, cytisine, and oxymatrine | [116] | |
| ESCs | Human | hESCs | Neurotoxicity of nanoparticles | [80] |
| Mouse | mESCs | Developmental neurotoxicity | [117] | |
| Human | 3D neurospheres derived from hESCs | Developmental neurotoxicity | [118] | |
| ASCs | Human | AD-MSCs neural cells | Lead cytotoxicity | [119] |
| iPSCs | Human | hiPSC derived neurons | Cytotoxicity analysis of various materials | [120] |
| Human | hiPSCs derived neural cells | Neurotoxic response of different biomaterials | [121] | |
| ESCs | Human | ESC-derived fibroblasts | Cytotoxicity of mitomycin C | [122] |
| Mouse | ESCs-derived embryoid body | Developmental cytotoxicity test using embryoid bodies | [123] | |
| Mouse | ESCs-derived endothelial cells | Endothelial cell developmental cytotoxicity | [124] | |
| Human | ESC-derived fibroblasts | Genotoxicity of mitomycin C | [125] | |
| ASCs | Human | AD-MSCs | Genotoxicity of silver nanoparticles | [126] |
| Human | AD-MSCs | Effect of hexanoyl glycol chitosan on the proliferation of AD-MSCs | [127] | |
| Human | Stem cells from human exfoliated primary teeth (SHED) | Cytotoxicity of several of materials | [128, 129] | |
| Human | BM-MSCs and AD-MSCs | Metal-based materials biocompatibility analysis | [130] | |
| Mouse | BM-MSCs | Molecular cytotoxicity of hydroxyapatite nanoparticles | [131] | |
| Mouse | Spermatogonial stem cells | Reproductive cytotoxicity of hydroxyurea | [132] | |
| iPSCs | Human | iPSC-derived proximal tubular-like cells | Nephrotoxicity testing of drugs | [133] |
| Human | iPSCs | Developmental cytotoxicity of retinoid analogs | [134] | |
| Human | iPSC-derived 3D liver spheroid | Phenotypic characterization of cytotoxic compounds | [135] | |
| Human | iPSCs | Identification of embryotoxicity of thalidomide | [136] |
3.2. Cardiotoxicity studies
The heart is the most vital organ in the body and therefore, biomaterials and drugs developed for cardiomyopathies must be assessed for their toxic effects on the heart. Newly developed biomaterials and drugs may cause extreme cardiotoxicity as one of the study shows that almost 90% of newly developed materials are rejected in clinical trials before their application in pre-clinical trials and 45% are failed due to cardiotoxicity [137]. Stem cell-derived cardiomyocytes have been widely recommended for testing the cytotoxic effects of alkaloids of Sophora tonkinensis on cardiomyocytes [116].
In a study, hESC-derived cardiomyocytes were used by Braam et al to study the dose-dependent effects of 12 cardiac and noncardiac drugs on cell their cell viability and dose-response [109]. In another study, the LV structure was successfully preserved by alginate microbeads and hMSCs encapsulated in microbeads and avoided negative LV remodeling after myocardial infarction. Compared with PBS control or cells alone, cell survival was greatly improved in the encapsulated hMSC community. Microspheres and hMSCs decreased infarct region and increased arteriole formation. This proved the biocompatibility of alginate microspheres with hMSCs in cardiac damage [111]. Ma et al tested whether, relative to unregulated melatonin in vitro, the protective effect of melatonin encapsulated by poly(lactide‐co‐glycolide) (PLG)‐monomethoxy‐poly‐(polyethylene glycol) nanoparticles (melatonin nanoparticles [Mel-NPs]) on adipose-derived mesenchymal stem cells (ADSCs) was enhanced. They discovered that Mel-NPs decreased p53-cyclophilin D complex formation, inhibited the opening of mitochondrial permeability transfer pores, and recovered ADSCs from hypoxia/reoxygenation injury. In addition, in rat myocardial infarction regions, Mel-NPs can attain better ADSC survival rates than free melatonin, and the therapeutic benefits of pre-treated ADSCs with Mel-NPs became more evident [112].
Wang et al used hiPSC derived cardiomyocytes to check the effects of matrine, sophocarpine, cytisine, and oxymatrine by assessing cell viability and level of LDH release [116]. In table 2 further studies are presented to prove that stem cells are the best candidates for investigating the cardiotoxicity potential of biomaterials and drugs.
High throughput testing of biomaterials and drugs could also be performed on stem cells to conduct millions of experiments in a short time and thus quickly generate data for use in translational research. For instance, in one of study, beating-cardiomyocyte layer was tested in a high-throughput manner by treating the hiPSCs [138]. The work involved the use of 96 well plates equipped with sensors to measure the real-time impedance of each cell culture and develop the personalized medicine platform for well-known drugs used in cancer chemotherapy. This personalized medicine approach proved applicable and successful in checking the effects of doxorubicin [139] and trastuzumab [140] on cardiomyocytes. The process of development of personalized medicine approach consisted of the removal of the somatic cells from diseased patients and reprogrammed them into iPSCs. During the characterization of cardiomyocytes through specific assays, drugs were applied on these disease-specific cardiomyocytes for cardiotoxicity analysis [141].
3.3. Neurotoxicity studies
The central nervous system (CNS) is the most delicate and interconnected part of our body. Screening of biomaterials and drugs for neurotoxicity by in vitro trials is highly recommended before their application on animal models and humans in order to avoid their adverse effects on CNS [142]. Stem cells possess a huge capacity for differentiation into a neural stem cell (NSC) depending upon the application of a suitable stimulus needed for neural cell development. Newly formed NSCs can further develop into neurons and thus can be used in the cure of different diseases of CNS [143]. NSCS can also be treated with different drugs to find the suitability of these stem cells for the discovery of novel drugs that could treat CNS disorders [144].
In an interesting study, hESCs were treated with gold nanoparticles to predict the effects of these nanoparticles on the neural differentiation of cells [80]. Baek et al treated mESCs with neurotoxins to determine the dose-dependent effects of these materials [117] while in another study embryoid bodies (EBs) derived from mESCs were used to predict the developmental cytotoxicity of various materials [145]. In the context of in vitro studies, 3D neurospheres were used to screen the cytotoxic effects on the hESCs, by monitoring the changes in gene expressions associated with nervous system disorders [118]. In another study, AD-MSCs were treated with lead and the differentiation potential of MSCs into neural cells was evaluated [119]. Tukker et al measured the effects of various pharmaceutical compounds by the treatment of commercially available hiPSC derived neurons and found that these compounds affect the cell proliferation in a dose-dependent manner [120]. In a comparative neurotoxic study, the substantial differences, among different cell types like hiPSCs derived neural cells, neurons, hiPSCs, and astrocytes, in neurotoxic response were studied using 80 neurotoxic compounds [121]. Table 2 shows drug effects studies regarding neurotoxicity using stem cells.
3.4. Nephrotoxicity studies
Kidneys are the most delicate and a crucial part of our body and are involved in the purification of blood by removing waste materials present in the blood. In vitro screening of the cytotoxic effect of drugs on nephrons is highly recommended before their application in vivo studies to validate their safety before their applications on animals and humans [146, 147].
In terms of their ability to grow into fully functioning renal cells, hiPSCs have sparked tremendous interest in nephrotoxicity studies. These cells can produce any sort of cell in the human body and can be replicated quickly [148]. hPSCs are now being used to produce renal cells in vitro using combinations of growth factors and small molecules to mimic progression through normal embryonic kidney formation, including both ESCs and human iPSCs (hiPSCs) [149–151]. A two-step procedure was developed by Chuva et al in which kidney organoids were transplanted into a chick chorioallantoic membrane to provide a vascularized environment that is required for the organoids to mature further [152]. The results showed that as compared to 2D cultures, this model demonstrated improved sensitivity to reported nephrotoxic drugs [153]. Thirty compounds including 18 nephrotoxicants that are specifically toxic to renal proximal tubular cell (PTC) and 12 compounds that are not toxic to PTC were evaluated for nephrotoxicity prediction by evaluating the expression levels of IL6 and IL8. For this study, targeted cells (i.e. PTC-like cells were obtained from hiPSCs by Kandasamy et al. Their findings showed a higher test accuracy of PTC-like cells derived from hiPSC (87%) than human PTC (82%), suggesting that the use of PTC-like cells derived from hiPSC could allow improved prediction of high-precision proximal tubular cytotoxicity in humans [133].
3.5. Other cytotoxicity studies
The treatment of stem cells with different kinds of biomaterials and drugs may allow them to differentiate into various types of cell lineages which could be beneficial for screening the cytotoxicity of biomaterials in different disease conditions. For instance, Cao et al reported in a study that fibroblastic progenies derived from hESC are more sensitive to the mitomycin C cytotoxicity than human fibroblasts of the lung [122]. Embryoid bodies test (EBT) was developed by Kang et al to quantify the decrease in the EB region. During embryo development, a decrease in EB size maybe the result of the abnormal differentiation process. Compared to traditional Embryonic stem cell test (EST), as EBT is quite advantageous as it can be performed quickly and can be used in a high-throughput manner for determining the cytotoxicity of materials [123]. Festag et al developed an experimental protocol for differentiating mESCs into endothelial cells using EBs. The hanging drop process for EB generation and subsequent suspension culture for distinction are included in this protocol to test the endothelial development cytotoxicity [124]. In comparison with the fibroblastic cell line and primary cultures of peripheral blood lymphocytes, Vinoth et al demonstrated that hESC-derived fibroblastic progenies are susceptible to genotoxicity screening upon exposure to mitomycin C [125].
In the context of stem cell in vitro models, Ag-NP were tested for their cytotoxicity and genotoxic effects by Hackenberg et al by treating human adult-MSCs. Ag-NP-induced DNA disruption was detected at high exposure levels based on the results of the comet assay and the chromosomal aberration test [126]. Remya et al used mouse BM-MSCs to test the molecular cytotoxicity of hydroxyapatite nanoparticles by detecting the development of reactive oxygen species (ROS) and apoptosis linked to oxidative stress [131]. The application of mouse spermatogonial stem cells to predict in vivo male reproductive cytotoxicity was demonstrated by Jeon et al by demonstrating the effects of hydroxyurea on DNA damage, ROS development, and apoptosis [132].
Palmer et al studied that hiPSCs have been used for tracking biomarkers such as ornithine and cysteine, believed to be involved in metabolic processes for cell proliferation and differentiation during embryonic development, to assess the developmental cytotoxicity of various retinoids [134]. Sirenko et al developed a hiPSC-derived 3D liver spheroid system with the assessment of parameters including spheroid size and form, cell number and spatial distribution, nuclear characterization, cell viability, apoptosis, and mitochondrial capacity for in vitro high-throughput hepatotoxicity assessment by measuring 48 compounds (42 recognized toxic agents and six non-toxic chemicals). Of the 42 identified toxic compounds, 36 (86% sensitivity) exhibited cytotoxicity effects, while six non-toxic compounds showed no effect on cell viability and spheroid phenotypes (100% predictivity) [135]. Thalidomide developmental toxicity in human cells was first observed in the hiPSC-based in vitro EST method in a study by Aikawa et al with their new parameters despite the use of three mouse embryonic stem cell test (mEST) endpoints (cytotoxicity to hiPSCs, cytotoxicity to human dermal fibroblast, and inhibition of cardiac differentiation) [136]. Vital pulp methods need the applications of biomaterials for the formation of a protective layer over the exposed vital pulp indirect pulp capping and pulpotomy procedures [78]. The materials used as protective coatings should display enough bioactivity and biocompatibility to accelerate dental pulp stem cell activity and pulp healing in primary teeth.
Stem cells from human exfoliated primary teeth (SHED) obtained from dental pulp explants or by digestion of dental pulp tissue from exfoliated deciduous teeth exhibit immunosuppressive properties [154]. SHED can differentiate into multiple cells including osteoblasts, neurons, adipocytes and endothelial cells even though their origin is from the dental pulp [155].
The cytotoxicity of several materials such as calcium hydroxide (Ca(OH)2), eugenol with zinc oxide (ZOE), mineral trioxide aggregate (MTA), biodentine and Theracal LC was carried out to understand the cytotoxic effects of these materials on SHED [128, 129]. The results showed that application of calcium hydroxide ZOE, MTA and biodentine promoted the cell viability and migration of SHEDs while Theracal LC leads to loss of viability of SHEDs [156].
In another study, metal-based materials were functionalized with ascorbic acid and were supplemented with SiO2-coating at 0.1 and 0.4 M concentrations. Using both bone marrow and adipose-derived mesenchymal stem cells, the biocompatibility of the materials collected was tested in vitro. The research findings suggested a favourable effect of silica coatings doped with 0.4 M ascorbic acid on the proliferation rate of the investigated cells [130]. By loading ADSCs spheroids into a macroencapsulation mechanism composed of filtration membranes of polytetrafluoroethylene, a model system was constructed by Wang et al. The in vivo research was performed by embedding blank or ADSC-laden machines subcutaneously in rats. The ADSC-laden systems were better vascularized after 4 week implantation and caused slightly fewer fibrotic tissue development relative to the non-cellular controls [157]. In one of an interesting study, hPSC-derived human lung organoids (HLOs) were used to evaluate the effects of scaffolds fabricated from PLG, polycaprolactone, and poly (ethylene glycol) [78]. The results showed that pore size and mechanical properties of scaffolds have a major effect on the development of the epithelium of HLOs.
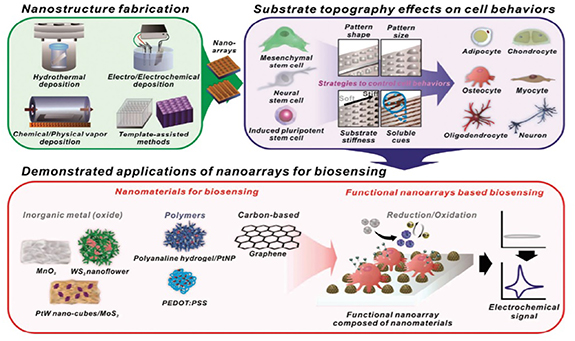
Jeong et al studied the effect of hexanoyl glycol chitosan (HGC) on the proliferation of AD-MSCs and its synergistic effect on the basic fibroblast growth factor (bFGF) was evaluated which has been commonly used to induce cell proliferation. They observed that, even at low bFGF concentrations, the presence of HGC improved the proliferative ability of AD-MSCs during long-term culture. In addition, the expression of genes associated with senescence was inhibited and mitochondrial functioning increased. Taken collectively, these results indicate that the capacity for sustained in vitro development of AD-MSCs is shown by the HGC [127]. The effects of nanostructures on the differentiation of stem cells are shown in figure 3.
Figure 3. Schematic illustration of functional nanoarrays for investigating stem cell fate and functions [106].
Download figure:
Standard image High-resolution image4. Organ-on-a-chip for cytotoxicity studies
Two-dimensional cell cultures models cannot give completely reliable results during biomedical material cytotoxicity screening studies as these cultures form monolayers on the surface of culture plates and offer only the upper layer of cells to come in contact with biomaterials [95]. The results obtained from 2D cell cultures, therefore, greatly vary from those obtained during in vivo studies due to the difference in the genetic makeup of animals and human beings. In order to develop systems that can replicate in vivo conditions, 3D cell culture systems have been developed, which uses human-derived cells and thus mimic the human cell biology to a certain extent but are not able to replicate the physiological environment of the body [94]. Therefore, scientists are moving towards bionic and more stable models for in vitro cytotoxicity analysis. Microfluidic chips were used for the analytical assay used in the lab but now it became the key player to develop better in vitro cytotoxicity testing models like organ-on-a-chip [158, 159]. Organ-on-a-chip, a subtype of microfluidic chips, includes cell to cell co-cultures and microenvironments that can be adjusted more accurately according to the function and growth of the cells cultured on the chip. Therefore, it can successfully mimic the physiology of human organs during in vitro studies [160].
Studies show that 2D cell cultures developed for evaluation of cytotoxicity of biomaterials for the liver cells exhibited certain shortcomings including loss of activity, reduced gene expression, and loss of liver-specific functions [161]. In order to overcome these shortcomings, scientists developed specific liver chips that mimicked the functionality of the liver as compared to 2D cell cultures. Bhise et al embedded HepG2 cells photo-crosslinked gelatin methacrylate hydrogel which formed spheroids. These were then arranged on a microfluidic chip to evaluate the cytotoxic effects of different biomaterials on the liver cells through gene expression studies. The liver chips remained functional for 30 d and were able to screen the cytotoxic effects of various materials on the liver cells [162]. In another study, Zuchowska et al checked the cytotoxic effects on the proliferation and viability of hepatic cells by treating 3D spheres culture of HepG2 cells with an anticancer drug named 5-FU. The study showed that an increase in the diameter of spheres facilitates the resistance of HepG2 cells towards 5-FU [163]. Jang et al constructed species-specific liver chips from the cells of humans, dogs, and rats to find how different biomaterials induce cytotoxic effects on liver cells. Treatment of species-specific liver chips with stool compounds exhibited species specific cytotoxic effects on the liver cells through the study of expression of various biomarkers, microscopic, and observations [164].
Nerve chips were developed to study the effects of various biomaterials on the viability and proliferation of kidney cells. Gramowski et al fabricated a nerve chip on the microelectrode array using rat cortical neurons via neural chip technology. Treatment of cells with three nanomaterials including nano TiO2, nano carbon black, and nano Fe2O3 produced cytotoxic effects on the nerve cells via interference with the electrical activity of nerves [165]. Nierode et al constructed a polystyrene microarray chip containing human neural progenitor cells which were embedded inside 532 microwells of microarray chip. The study was successful in screening the cytotoxic effects of 24 different compounds on neural cells using a polystyrene microarray chip based on fluorescence analysis [166]. Gill et al used neurospheres made via Sprague Dawley rat NSCs to assess the cytotoxic effects on the cell viability of NSC by application of nine different compounds. These compounds reduced the size of spheres on the chip displaying cytotoxic effects on the cell viability of NSCs [167].
The heart is regarded as of the most vital organs of the body but during biomaterials screening assays, serious issues of cytotoxicity on the cell viability of cardiomyocytes may arise. In order to improve the performance of biomaterials screening models, heart chips were fabricated instead of the use of animal models and 2D cell cultures for obtaining promising results. Qian et al used a cardiac chip consisting of cardiomyocytes derived from hiPSCs. The electrophysiology and contraction of cardiomyocytes were observed by the application of different biomedical materials [168]. Caluori et al prepared a heart chip embedded with electro-mechanical biosensors having the ability to detect cardiac excitation-contraction coupling. They tested the cytotoxic effects of verapamil and isoproterenol, and their dosage–response on cardiomyocytes and proved that this heart chip is the best model for studying the cytotoxic effects on cardiomyocytes [169]. Ville et al constructed a heart chip using hiPSC-derived cardiomyocytes for the application of measuring electrophysiological data. Treatment of heart chips with different dosages of drugs exhibited a varied cytotoxic response exhibiting the effectiveness of microchips in the evaluation of biomaterials screening [170].
Organ-on-a-chip is a great asset these days for studying the cytotoxic effects of different biomaterials mimicking in vivo conditions and providing a friendly environment for cells viability and quick response to biomedical materials. Nevertheless, more studies with better organ-on-chip are needed to enhance the reproducibility of results and their comparison with clinical data.
5. Prospects and challenges
Despite the unique advantages and potential applications, stem cell-based 2D cell culture in vitro approaches cannot completely substitute in vivo animal models as they do no match with the histology and molecular biology of complex living tissues in animals and humans [171]. Lack of systemic metabolism in 2D cultures and the incapability of long term monitoring biomaterials make in vitro stem cell culture systems less useful for systemic cytotoxicity studies of biomaterials [172, 173]. Thus, there is a dire need for the development of dependable 3D cell culture models that properly could depict animal and human systems, and assessment of adverse effects of biomaterials on humans [174]. Human stem cell-based 3D systems used for cytotoxic studies have their advantages like self-renewal ability of stem cells, no interspecies variability, and their potential to differentiate into various cell lineages [20, 175]. However, unlike in normal cell culture systems, in addition to the changes in cell viability or cell death due to the biomaterial to be tested, the effect on pluripotency should also need to be taken into consideration [176]. Quantitatively determining the differentiation potential of stem cells during treating with material is rather complex compared to either viability or toxicity assessment. The use of somatic cells derived from hESCs, for studying the cytotoxicity of biomedical materials, has paved its way in functional toxicology by validating the experiments thus making them a potential system in cytotoxicity and biocompatibility analysis. iPSCs had become popular because of the fact they the show same properties as naturally occurring stem cells and their applications in cytotoxicity studies have made them a centre of interest to be used for cytotoxic analysis [136, 177, 178]. Moreover, iPSCs do not have ethical barriers as compared to ESCs and can be effectively used as a potential tool for predictive toxicology and biocompatibility of biomaterials [179–182]. There are certain limitations regarding iPSCs based models including the unwanted differentiation of stem cells into cancer lineages and high cost which still need to be solved [136, 177, 178].
Organ-on-a-chip platforms are highly promising for studying the cytotoxic effects of different biomaterials in an in vivo mimicking condition and can provide relatively quick results [95]. However, such systems still need to be fine-tuned by incorporating multiple types of native cells such as macrophages, vascular cells, and other stromal cells along with specific stem cells of interest.
On other hand, stem cell systems do not provide many promising results while checking the sensitivity and biocompatibility of biomaterials. Therefore, there is a dire need to enhance the sensitivity to stem cell-based cytotoxicity studies which help in less usage of animal models. Nonetheless, stem cell systems prove to be a potential and worthy tool for cytotoxicity studies and biocompatibility testing of biomedical materials, but advances should be made in the future to develop much better in vitro cytotoxic assays based on stem cell by overcoming the current limitations and disadvantages of such systems.
Acknowledgments
This article was made possible by NPRP10-0120-170211 Grant funded by the Qatar National Research Fund (a part of Qatar Foundation). Statements made herein are solely the responsibility of the authors.
Conflict of interest
All authors confirm that no conflicts of interest exist.