Abstract
Increasing temperatures and decreasing precipitation in large areas of the planet as a consequence of global warming will affect plant growth and survival. However, the impact of climatic conditions will differ across species depending on their stomatal response to increasing aridity, as this will ultimately affect the balance between carbon assimilation and water loss. In this study, we monitored gas exchange, growth and survival in saplings of three widely distributed European pine species (Pinus halepensis, P. nigra and P. sylvestris) with contrasting distribution and ecological requirements in order to ascertain the relationship between stomatal control and plant performance. The experiment was conducted in a common garden environment resembling rainfall and temperature conditions that two of the three species are expected to encounter in the near future. In addition, gas exchange was monitored both at the leaf and at the whole-plant level using a transient-state closed chamber, which allowed us to model the response of the whole plant to increased air evaporative demand (AED). P. sylvestris was the species with lowest survival and performance. By contrast, P. halepensis showed no mortality, much higher growth (two orders of magnitude), carbon assimilation (ca. 14 fold higher) and stomatal conductance and water transpiration (ca. 4 fold higher) than the other two species. As a consequence, P. halepensis exhibited higher values of water-use efficiency than the rest of the species even at the highest values of AED. Overall, the results strongly support that the weaker stomatal control of P. halepensis, which is linked to lower stem water potential, enabled this species to maximize carbon uptake under drought stress and ultimately outperform the more water conservative P. nigra and P. sylvestris. These results suggest that under a hotter drought scenario P. nigra and P. sylvestris would very likely suffer increased mortality, whereas P. halepensis could maintain gas exchange and avoid water-induced growth limitation. This might ultimately foster an expansion of P. halepensis to higher latitudes and elevations.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence.
Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
Global warming is rapidly changing climatic conditions worldwide, altering plant-species survival and performance (Lloyd and Bunn 2007, Allen et al 2010). In particular, increases in temperature, coupled with reductions in rainfall, are exacerbating aridity in large areas of the globe, thereby augmenting air evaporative demand and the so-called hotter drought (sensu Allen et al 2015). Given that species differ in drought sensitivity (Baltzer et al 2008, Kursar et al 2009), the increase in aridity may affect species persistence and filter community composition. This can lead to sweeping changes in forest-regeneration patterns and ultimately biodiversity distribution (Allen and Breshears 1998, Jump and Peñuelas 2005). In fact, recent major episodes of forest die-back attributed to drought have been reported around the planet (Bigler et al 2006, Allen et al 2010, Kharuk et al 2013), and the impact of droughts is expected to increase in the future (Allen et al 2015). Therefore, detailed knowledge of the response of sympatric tree species to more severe aridity is crucial for predicting the future composition of the forests.
Stomatal control, i.e. the sensitivity of stomatal closure to the increase in air evaporative demand and plant water stress, is involved in the immediate response of plants to drought (Lambers et al 2008). The impact of air evaporative demand on stomatal conductance differs between species (Tardieu and Simonneau 1998, Mediavilla and Escudero 2003a), which has implications for plant performance under drought conditions. For a given level of air evaporative demand, species with a strong stomatal control (i.e. stomata close at low or moderate air evaporative demand) will reduce transpiration and the risk of hydraulic failure but at the cost of reducing carbon assimilation. In contrast, species with weak stomatal control (i.e. stomata close at high air evaporative demand) will keep high transpiration, which might increase the risk of hydraulic failure (McDowell et al 2008). Although the sensitivity of stomatal control and embolism vulnerability are interrelated and their role in plant drought tolerance is currently under debate (Garcia-Forner et al 2016a, 2016b, Martínez-Vilalta and Garcia-Forner 2017), it is widely accepted that stomatal control sensitivity is related to the plant strategy to cope with drought (Martínez-Vilalta et al 2014). The interspecific differences in stomatal closure have been commonly simplified into two categories: Species with weak (isohydric) vs. strong (anisohydric) stomatal control (Klein 2014). Taxonomic groups are often defined within these categories, and pine species in particular are considered isohydric species compared to other taxa such as oaks or junipers (Zweifel et al 2007, Meinzer et al 2014). However, the stomatal control at the species level may differ among closely related taxa (White et al 2000, Héroult et al 2013), and even within species (Schultz 2003, Sade et al 2012). Therefore, precise knowledge of the stomatal mechanisms used to cope with drought across related plant species is necessary to predict their response to climate change.
The distribution of pine species native to Europe is segregated along altitudinal gradients in a predictable manner (Richardson 2000) suggesting the existence of environmental factors that differentially influence their distribution. Some species thrive in warm, southern Mediterranean areas with low rainfall (e.g. P. halepensis) while other species live in high-mountain or boreal environments with low evaporative demand (e.g. P. sylvestris). In fact, the effect of increasing aridity on European pine species appears to differ (Peñuelas et al 2001, Sánchez-Salguero et al 2012, Herrero et al 2013, Matías et al 2017), and this differential effect is expected to trigger shifts in pine distributions (García-Valdés et al 2013, 2015).
The role of stomatal control on drought tolerance can be conflated with embolism resistance when this trait differs between species (e.g. junipers vs. pines; Garcia-Forner et al 2016a, Martínez-Vilalta and Garcia-Forner 2017). However, for closely related species that exhibit similar xylem embolism vulnerability, which seems to be the case in pine species (Delzon et al 2010, Choat et al 2012), a different stomatal control in response to drought can determine drought tolerance differences. Thus, pines with a strong stomatal control could lose less water than those with a weak stomatal control, but at the expense of lower photosynthesis (McDowell et al 2008, Matías et al 2017). Therefore, gas exchange and performance in general could be more limited by water scarcity in pines with a conservative strategy to cope with water stress.
Measurements of gas exchange are usually performed at the leaf level due to, among other reasons, the speed and versatility of these types of measurements, together with the difficulty (or impossibility) in many cases of sampling at the whole-plant level (Medrano et al 2010). However, leaf-level data have the drawback that it is difficult to extrapolate results to the whole plant and, thus, to have measurements of performance that are representative at the plant level, including net assimilation and water loss (Escalona et al 2003, Medrano et al 2012, 2015). Transient-state closed chambers that enable measurements at the whole-plant level can overcome this limitation (Reicosky and Peters 1977, Pérez-Priego et al 2010). Such chambers enclose the plant for the monitoring of CO2 uptake and H2O emission in order to calculate net photosynthesis, transpiration, stomatal conductance, and water-use efficiency for the whole aboveground part of the plant (Pérez-Priego et al 2010). A combination of measurements at the leaf and whole-plant level should allow a detailed and realistic characterization of the response of species to increasing water stress.
The aim of this study was to analyse the consequences of stomatal control under dry conditions for the performance of three ecologically contrasted European pine species (P. halepensis, P. nigra and P. sylvestris). These trees are major components of European forests, and their response under a scenario of increasing aridity might have strong implications for forest composition and regeneration across the continent (Poyatos et al 2013, Herrero et al 2013). For this purpose, we conducted a common garden experiment with even-aged saplings, monitoring survival and growth for two years. In addition, we measured stem-water potential plus CO2 and H2O exchange both at the leaf level with leaf cuvettes as well as at the plant level using a transient-state closed chamber. We hypothesized that there would be differences in stomatal behaviour in response to dry conditions among species of a genus usually considered isohydric such as Pinus (hypothesis 1). A strong stomatal control is usually associated with a high control of water potential. However, a weak stomatal control will sustain higher photosynthesis and transpiration under dry conditions. Thus, we hypothesized that within closed related isohydric species, increased gas-exchange (and consequently plant growth) at whole-plant level will be tightly linked to low plant-water potential (hypothesis 2). Given that stomatal regulation and transpiration are driven by differences in water content between the plant and the atmosphere, we hypothesized that the survival and growth of the different species will be mediated by their response to air evaporative demand (hypothesis 3). Overall, we seek to determine the role of gas exchange and stomatal regulation in the species drought sensitivity differences. This will enable more accurate predictions concerning the response of these species under increasing aridity and the potential shifts in species distribution areas.
Material and methods
Species and plant material
The study species were P. halepensis Mill., P. nigra Arnold, and P. sylvestris L, three pines with a broad distribution in Europe and exposed to contrasting climatic conditions. In southern Europe, these species segregate clearly along an aridity gradient in the order P. halepensis > P. nigra > P. sylvestris, whereas tolerance to low temperatures follows the opposite trend (P. sylvestris > P. nigra > P. halepensis; Tapias et al 2004, Ruiz de la Torre 2006, Fernández-Pérez et al 2018, appendix S1 available at stacks.iop.org/ERL/13/045004/mmedia). P. halepensis is distributed throughout the Mediterranean basin from sea level to 1200 m a.s.l., P. nigra from 800–2000 m a.s.l. (Mediterranean basin and the Alps) and P. sylvestris from 1000–2100 m a.s.l. (Boreo-Alpine/Eurosiberian distribution) with its southernmost distribution area in Granada, southern Spain (Castro et al 2004, elevational ranges for southern Europe; Richardson 2000). Seeds from the four species were collected when ripe from certified provenance regions of the Iberian Peninsula. The seeds were stored under cold, dry conditions until sowing. Seeding was done in winter 2012 using 300 m L plastic containers filled with fertilized peat (White 420 F6 Kekkilä, Finland; pH 4.7) containing 0.8–1 kg m−3 of a slow-release fertilizer NPK 16-10-20. Plants were initially grown in a greenhouse of the Centro Nacional de Recursos Genéticos Forestales 'El Serranillo' (Guadalajara, Spain, 40.665594°N, 3.170889°W) to avoid frost damage. In mid-May 2012, the plants were moved outdoors and cultivated under optimal conditions until 15 February 2013, when they were transferred to the common garden site (one year old by that time).
Study site and experimental set up
The study was conducted in a common garden experiment at 'Huerta de La Paloma' farm, located in Granada (37.167619° N, 3.616056° W; S Spain) on flat (slope ca. 2%), agricultural terrain at 649 m a.s.l. Plants were planted in mid-February 2013 at a distance of 1.25 m from each other following a randomized-block design with three blocks (70 individuals per block and species; blocks separated each other by 2.5 m), and randomly distributed within each block. The initial size of the plants (stem height and root-collar diameter) was measured just after planting to calculate the final growth increment, initial values being 14.29±0.16 cm for P. halepensis, 6.80 ± 0.13 for P. nigra, and 4.36 ± 0.09 for P. sylvestris for leader shoot length; and 3.35 ± 0.03 mm for P. halepensis, 3.46 ± 0.04 for P. nigra, and 2.87 ± 0.04 for P. sylvestris for trunk diameter (significant differences among species, P < 0.0001 for both variables).
The climate in the area is Mediterranean, with hot, dry summers and precipitation concentrated in autumn and spring. The mean annual rainfall is 394 ± 38 L m2 and the mean temperature is 15.3 °C ± 0.1 °C, with a mean maximum of the hottest month of 35.7 °C ± 0.2 °C and a mean minimum of the coldest month of −0.1 °C ± 0.2 °C (period 2006–2015; climatic data from a meteorological station located at IFAPA Research Field Station, a place with characteristics identical to those of the study site located 1.6 km away). These climatic conditions would be drier and hotter than those naturally encountered by P. nigra and P. sylvestris (Barberó et al 2000, Matías and Jump 2012, Enescu et al 2016, Houston-Durrant et al 2016, appendix S1), whereas they fall within the ecological range of P. halepensis (Barberó et al 2000, Mauri et al 2016, appendix S1). The soil is deep with a loamy texture, and average values of 44.8% sand, 41.8% silt and 13.3% clay (mean for the profile up to 1 m depth; no marked horizons present in this profile).
Plant survival and growth
Plant survival and growth were sampled at the end of the second growing season after planting (September 2014, thus 3 yr old plants) for all the saplings in the experimental plot (n = 630). Stem volume was calculated for each year assuming a conical shape for the stem, with basal diameter given by the data of stem-root collar (average of two perpendicular measurements) and height given by the height of the plant.
Figure 1. Schematic representation of the experimental design. A common garden experiment was performed in a flat agricultural terrain where saplings of the three study species (P. halepensis, P. nigra and P. sylvestris) were randomly distributed. Three campaigns with seven rounds of measurements were performed. At each round, gas exchange at whole-plant level along with ambient and canopy temperature were monitored for a maximum of 180 sec in the selected four individuals per species. Measurements of gas exchange were made using a transient-state closed chamber connected to a gas analyser, whilst measurements of temperature were made by means of a thermocouple and an infrared thermometer installed in the chamber. In each measurement, the chamber was engaged into a frame previously installed around the sapling to avoid soil respiration. Moreover, measurements of edaphic humidity were taken in each campaign from access tubes installed next to each sapling. Plants were initially at a distance of 1.25 m, but those used for gas exchange measurement with the transient-state closed chamber two years after planting were at a higher distance (≥2.5 m) due to events of mortality through the experiment.
Download figure:
Standard image High-resolution imageGas exchange at the leaf level
Net photosynthesis (An), stomatal conductance (gs) and mid-day stem-water potential () were measured for a subsample of 27 randomly selected saplings (nine per block) per species on 9th June and 21st July of the second growing season after planting (year 2014; only measured in July). CO2 and H2O fluxes were measured by means of an infrared gas analyser (IRGA, LI-6400) coupled to a conifer chamber model LI-6400-05 (Li-Cor Inc., Lincoln, NE, USA). Gas exchange (CO2 and H2O evolution in the conifer chamber) was registered every 5 sec for a period of 90 sec after a constant rate had been reached. Intrinsic water-use efficiency (iWUE) was calculated as the ratio between An and gs. We used iWUE instead of water-use efficiency (WUE = An/transpiration rate) at leaf level because air evaporative demand can interfere on WUE through its effect on transpiration (i.e. the same leaf may have lower WUE when the environment is drier; Medrano et al 2010). This effect was considered on whole-plant measurements monitoring the same individuals over the day. In addition, was measured at midday with a pressure chamber (SKPM 1400, Skye Instruments, UK) in lateral twigs. Individuals were sampled between 12:00 and 16:00 h (solar time).
Gas exchange at the whole-plant level
An and transpiration (E) were also measured at the whole-plant level with a transient-state closed chamber. The chamber consisted of a translucent sheet stretched and fixed to a cubic aluminium frame structure (0.5 × 0.5 × 0.6 m) using adhesive tape. It had a thermocouple (Type T, 0.5 mm diameter) and an infrared thermometer (IRTS-P, Apogee, UT, USA), both installed near the chamber ceiling for measurements of air and canopy temperatures respectively (Ta, Tc), and the whole chamber was connected to an infrared gas analyser (IRGA LI-840, Li-Cor Inc., Lincoln, NE, USA) for gas-exchange measurements (figure 1; see Pérez-Priego et al (2015) for further details on chamber design). Four individuals of each species were monitored on three field campaigns carried out on 7th August, 12th September, and 21th October of 2014. A total of seven measurement rounds from predawn to sunset were performed per date. In each round, we monitored gas exchange of the 12 individuals sequentially. Molar fractions of CO2 and H2O along with air and canopy temperature were monitored at 1 Hz for each individual over 180 s. The individuals monitored were from the same block for logistic reasons (it was not possible to move the chamber with the associated net of electrical wire cables across the whole plot).
Other meteorological variables such as atmospheric pressure and total radiation were recorded from the meteorological station of the IFAPA Research Field Station. We used atmospheric pressure to calculate CO2 and H2O fluxes, relative humidity (RH) and vapour-pressure deficit (VPD). Photosynthetic active radiation (PAR) was estimated from total radiation by means of a conversion factor of 0.8667 developed for this region by Alados et al (1996). Moreover, soil-water content was measured for each campaign at 10, 20, 30, 40, 60, and 100 cm depth using the PR-2/6 Soil Moisture Profile Probe (Delta T, Cambridge, UK). We installed access tubes beside each individual (thus 12 sampling points in total) before the onset of transient-state closed-chamber measurements.
Flux calculations in the transient-state closed chamber
Fluxes were calculated from the initial slopes of CO2 and H2O molar fractions of the confined air vs. time, by using either linear or quadratic regression according to the fit (Pérez-Priego et al 2010). The raw values of CO2 molar fractions were previously corrected for the dilution effect (Hubb 2012). CO2 and H2O fluxes were calculated by means of bootstrapping with the purpose of reducing the uncertainty caused by the chamber (Wutzler and Priego 2014, Pérez-Priego et al 2015). Stomatal conductance was then estimated following Jones (2014), while WUE and iWUE were calculated as the ratio of photosynthesis to transpiration and stomatal conductance rates, respectively (Medrano et al 2010). See appendix S2 for further details about flux calculations.
Data analysis
All analyses were performed using R, version 3.3.2 (R Core Team 2016). We ran a principal component analysis (PCA) to summarize the environmental variables measured into independent axes. Soil moisture did not differ across species and did not change across rounds (appendix S3), and was therefore not included in the PCA. For the rest of the variables, we made a selection according to the degree of multicollinearity, estimated as the variance inflation factor (VIF; Harrel 2001). The selected variables were PAR, VPD and RH, which resulted in an axis that explained 82.94% of the environmental variance (PC1; eigenvalue of 2.49; Appendix S4). Vapour-pressure deficit and relative humidity were the variables with the most weight on the axis (35.01 and 34.35%, respectively), while PAR had the lowest weight (30.63%). Moreover, VPD and PAR were positively related to this axis, in contrast with RH (Appendix S4). PC1 was therefore considered a proxy for aridity (air evaporative demand [AED] hereafter) to assess the association between climate and ecophysiological performance in further analyses.
To analyse the association between AED and plant-performance variables we used generalized linear mixed models, controlling for the variation attributable to differences among species and individual saplings (glmm; Bates et al 2015, Pinheiro et al 2016). In these tests, 'species' was considered as a fixed factor while 'sapling' was considered to be a random factor. We accounted for nonlinear relationships between response variables and climate including a quadratic term (polynomial degree 2) in all models. In some cases, both the quadratic term and AED⁎species interaction were significant, indicating that the relationship between response variable and AED followed a quadratic function whose shape differed among species. In such instances, we considered as a threshold level the value of AED at which occurred an inflection in the response variable (i.e. inflection or turning point of a quadratic function). Differences in this threshold were considered a proxy for the sensitivity to AED at the species level. In every case, those species in which the threshold (i.e. the inflection point) was located at lower values of AED were considered more sensitive to aridity. Additionally, we also tested for differences among species in survival, growth, water potential, and gas exchange at the leaf level using glmm for all variables except survival, for which we used a mixed-effects Cox model. In these analyses, 'block' was considered as a random factor (Therneau and Grambsch 2000, Therneau 2015).
Error distribution and link functions were selected based on AIC. Full models including all variables were under the risk of over-parameterization, and in any case their interpretation was unwieldy, so we proceeded to simplify them beginning with the highest-order interactions and hierarchically moving on to single factors. We determined the effect of factors and interactions on response variables by testing whether the reduction in deviance following the elimination of a specific factor or interaction was significant using likelihood ratio tests (LRT; Crawley 2012). Once the model without non-significant factors and interactions was built (minimal adequate model or MAM), we assessed its goodness of fit and then checked for differences among factor levels by changing the reference level (i.e. with a directed contrast approach).
Results
Plant survival and growth
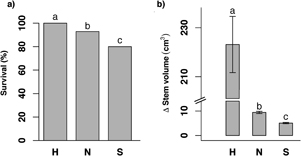
Survival differed among species (P < 0.0001), with an overall value of 100% for P. halepensis, 92.9% for P. nigra, and 80% for P. sylvestris (figure 2(a)). Stem-volume increment also differed among species (P < 0.0001), with a much higher value for P. halepensis followed by P. nigra and finally P. sylvestris (figure 2(b)).
Figure 2. Mean values of percentage of survival and increment of stem volume per species. Different letters denote significant differences between species. Error bars represent ±SE. Abbreviations: H = P. halepensis; N = P. nigra; S = P. sylvestris.
Download figure:
Standard image High-resolution imageAt a species level, survival at the end of the experiment (October 2014) was positively associated with the increment of stem volume during the first growing season (2013) in P. nigra and P. sylvestris (P = 0.0015 and 0.0019, respectively), meaning that bigger trees were less likely to die within species. This association was inexistent for P. halepensis given that there was no mortality in this species.
Gas exchange at the leaf level
At the leaf level, An registered the highest values for P. sylvestris in June, but similar values across species in July (table 1). Moreover, P. sylvestris showed a sharp decrease in An from June to July (P = 0.00032), whereas the other two species showed no differences between periods (P > 0.1 in both cases). Also, gs differed across species both in June and July (table 1), with P. sylvestris showing again a sharp decrease from June to July (P = 0.0014) while the other two species kept a similar value across periods (P > 0.2 in both cases). This resulted in maximum values of iWUE for P. sylvestris on both dates, while no differences appeared between P. halepensis and P. nigra (table 1). Midday (measured in July) was the lowest in P. halepensis, whereas there were no significant differences between P. nigra and P. sylvestris (table 1).
Table 1. Mean values ± SE of midday water potential and gas-exchange parameters measured at the leaf level for the three pine species in the second year after planting. Chisq/F and P-values of the 'species' factor are shown for each variable. Values of P < 0.05 are shown in bold, while differences between species are shown with different superscripts. An = Maximum net photosynthetic rate; gs = Stomatal conductance; iWUE = Intrinsic water-use efficiency; = Midday water potential.
| Variables | Date | Species | Chisq/F | Pr(>Chisq/F) | ||
|---|---|---|---|---|---|---|
| P. halepensis | P. nigra | P. sylvestris | ||||
| An | June | 2.84 ± 0.17a | 3.50 ± 0.28a | 4.3 ± 0.3b | 17.99 | 1.24E–04 |
| (μmol CO2 m2 s−1) | July | 3.4 ± 0.3a | 3.3 ± 0.4a | 2.85 ± 0.25a | 2.25 | 3.25E–01 |
| gs | June | 40 ± 3a | 55 ± 5b | 51 ± 4ab | 7.22 | 2.70E–02 |
| (mmol H2O m2 s−1) | July | 49 ± 3a | 56 ± 5a | 30 ± 3b | 17.93 | 1.28E–04 |
| iWUE | June | 73.5 ± 2.6a | 74.1 ± 3.0a | 97.4 ± 4.12b | 28.08 | 7.98E–07 |
| (μmol CO2/mol H2O) | July | 78.90 ± 7.16a | 68.3 ± 4.7a | 102.0 ± 5.7b | 21.91 | 1.74E–05 |
| (MPa) | July | −2.37 ± 0.04a | −1.88 ± 0.05b | −1.84 ± 0.04b | 57.25 | 3.70E–13 |
Gas exchange at the whole-plant level
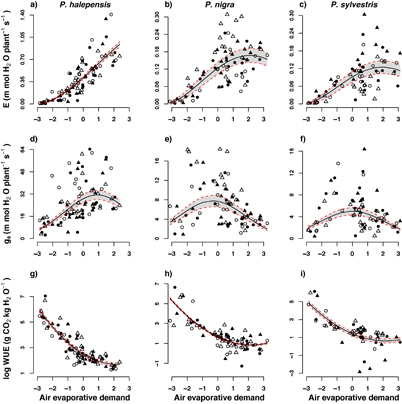
An, E, gs, WUE and iWUE at whole-plant level were much higher for P. halepensis than for the rest of species (table 2; figure 3), whereas P. sylvestris tended to have the lowest values (figure 3). E and gs were associated with AED following a quadratic function (i.e. bell-shaped relationship; P < 2 E–16 for quadratic term in both cases; table 3). These relationships differed between species (P < 0.003 for AED⁎species interaction term in all cases). For both variables, P. halepensis showed a greater initial reaction (steeper initial slope) under increasing AED as supported by estimates of interaction terms in P. nigra and P. sylvestris (−2.64 and −2.72 for E, −6.29 and −4.78 for gs, being P. halepensis the reference level; table 3, figures 4(a)–(f)). For gs, the value of AED at which the inflection point occurred followed the order: P. sylvestris < P. nigra < P. halepensis. In the case of E, P. halepensis did not show an inflection point (i.e. no reductions of transpiration at any level of AED). Lastly, An, WUE and iWUE showed a decrease with increased AED, with a similar pattern for all three species (non-significant interaction between AED and species; tables 2 and 3; figures 4(g)-(i).
Table 2. Results of the mixed models for the effect of air evaporative demand (AED; PC1 from PCA analysis) and species on the different response variables. Quadratic terms are indicated as '^2'. Reductions of deviance without each factor or interaction are shown for each variable. Values of P < 0.05 are shown in bold. An = Maximum net photosynthetic rate; E = Transpiration; gs = Stomatal conductance; WUE = water-use efficiency; iWUE = Intrinsic water-use efficiency.
| Response | Factor/Interaction | D.F. | ∆ Deviance | P value |
|---|---|---|---|---|
| An | Species | 2 | 51.60 | < 2.2 E–16 |
| AED | 1 | 0.44 | 1.14 E–02 | |
| AED^2 | 1 | 0.11 | 2.04 E–01 | |
| Species x AED | 2 | 0.06 | 6.39 E–01 | |
| Species x AED^2 | 2 | 0.05 | 6.82 E–01 | |
| E | Species | 2 | 32.51 | 8.71 E–08 |
| AED | 1 | 170.01 | < 2.2 E–16 | |
| AED^2 | 1 | 30.27 | 3.75 E–08 | |
| Species x AED | 2 | 119.26 | < 2.2 E–16 | |
| Species x AED^2 | 2 | 0.33 | 8.47 E–01 | |
| gs | Species | 2 | 33.64 | 4.95 E–08 |
| AED | 1 | 3.44 | 6.36 E–02 | |
| AED^2 | 1 | 51.06 | 8.94 E–13 | |
| Species x AED | 2 | 16.94 | 2.09 E–04 | |
| Species x AED^2 | 2 | 0.86 | 6.51 E–01 | |
| WUE | Species | 2 | 21.80 | 1.85 E–05 |
| AED | 1 | 202.86 | < 2.2 E–16 | |
| AED^2 | 1 | 43.41 | 4.44 E–11 | |
| Species x AED | 2 | 0.10 | 9.53 E–01 | |
| Species x AED^2 | 2 | 0.40 | 8.18 E–01 | |
| iWUE | Species | 2 | 17.93 | 1.28 E–04 |
| AED | 1 | 23.69 | 1.13 E–06 | |
| AED^2 | 1 | 14.83 | 1.18 E–04 | |
| Species x AED | 2 | 1.36 | 5.08 E–01 | |
| Species x AED^2 | 2 | 0.42 | 8.11 E–01 |
Figure 3. Mean values of net photosynthetic rate (An), transpiration rate (E), water-use efficiency (WUE), intrinsic water-use efficiency (iWUE) and stomatal conductance (gs) in sapling of P. halepensis, P. nigra, and P. sylvestris measured with a transient-state closed chamber. Mean values with rounds (1–7) and campaigns (August, September and October) are pooled. Different letters denote differences between species. Error bars represent ± SE.
Download figure:
Standard image High-resolution imageTable 3. Fixed-effects terms of mixed-effects models for the effect of air evaporative demand (AED; PC1 from PCA analysis) and species on the different response variables. We used lineal or quadratic terms ('^2') to model the relationship between AED and physiological parameters. The selection between these models was based on their significance (Table 2). Quadratic models responded to the equation Response = a + bx + cx2, where a is the intercept, b is the coefficient of the linear term, and c is the coefficient of the quadratic term. Interactions between species and AED (linear or quadratic) means that the relationship between AED and the response variable change across species. Pinus halepensis is the reference level in all cases. Thus, negative values of the interaction coefficients (Species x AED) indicate a different effect of AED on the response variable in P. nigra and P. sylvestris with respect to P. halepensis. For example, negative estimates of SpeciesN x AED and SpeciesS x AED terms are indicative of higher transpiration in P. halepensis and lower in P. nigra and P. sylvestris with increasing AED. Estimates and SE are transformed according to the transformation applied to each outcome. An, E, gs, WUE and iWUE as in table 2.
| Response | Factor/Interaction | Estimates | P value |
|---|---|---|---|
| An | Intercept | 1.437 ± 0.029 | <2 E–16 |
| SpeciesN | −0.918 ± 0.043 | <2 E–16 | |
| SpeciesS | −1.032 ± 0.044 | <2 E–16 | |
| AED | −0.031 ± 0.012 | 1.21 E–02 | |
| E | Intercept | 0.629 ± 0.019 | <2 E–16 |
| SpeciesN | −0.304 ± 0.027 | <2 E–16 | |
| SpeciesS | −0.351 ± 0.027 | <2 E–16 | |
| AED | 4.005 ± 0.185 | <2 E–16 | |
| AED^2 | −0.519 ± 0.096 | <2 E–16 | |
| SpeciesN x AED | −2.643 ± 0.246 | <2 E–16 | |
| SpeciesS x AED | −2.717 ± 0.242 | <2 E–16 | |
| gs | Intercept | 3.125 ± 0.111 | <2 E–16 |
| SpeciesN | −1.346 ± 0.157 | <2 E–16 | |
| SpeciesS | −1.753 ± 0.157 | <2 E–16 | |
| AED | 5.183 ± 1.173 | <2 E–16 | |
| AED^2 | −4.284 ± 0.612 | <2 E–16 | |
| SpeciesN x AED | −6.291 ± 1.556 | 1.00 E–04 | |
| SpeciesS x AED | −4.779 ± 1.545 | 2.20 E–03 | |
| WUE | Intercept | 2.799 ± 0.142 | <2 E–16 |
| SpeciesN | −0.857 ± 0.203 | 2.30 E–03 | |
| SpeciesS | −1.161 ± 0.207 | 3.00 E–04 | |
| AED | −16.84 ± 0.85 | <2 E–16 | |
| AED^2 | 5.938 ± 0.846 | <2 E–16 | |
| iWUE | Intercept | 4.454 ± 0.146 | <2 E–16 |
| SpeciesN | −0.739 ± 0.209 | 6.30 E–03 | |
| SpeciesS | −1.063 ± 0.212 | 7.00 E–04 | |
| AED | −4.183 ± 0.835 | <2 E–16 | |
| AED^2 | 3.3 ± 0.831 | 1.00 E–04 |
Discussion
In this study, we observed a link between stomatal response to air evaporative demand and plant performance under water stress in three pine species with contrasting ecology.
In accordance to our first hypothesis, species differed in their stomatal control under drought conditions. Moreover, the results support the contention that under increased evaporative demand, stomatal control might limit the performance of the high-mountain species (P. nigra) and particularly the boreal-alpine P. sylvestris, while the Mediterranean P. halepensis will cope better with drier and hotter conditions (hypothesis 3). These results have implications for understanding present and future species distribution in southern Europe, where aridity is expected to intensify under current climatic change.
Measurements at the leaf level suggest a high stomatal control in P. sylvestris and, to a lesser extent, in P. nigra under water stress. P. sylvestris did not show differences of stomatal conductance with the rest of species in June (when drought conditions were mild), but in July, under harsher conditions, it showed the lowest values. This is consistent with the photosynthesis results, i.e. P. sylvestris showed the highest values in June, but in July there were not significant differences between species. This is explained by the significant decrease in gs and An from June to July only in P. sylvestris. Therefore, it seems that higher conductance enabled maintenance of high photosynthetic activity in P. sylvestris earlier in the summer, an that both parameters plummeted concomitantly as aridity increased (see Hernández-Clemente et al 2011, Manzanera et al 2016 for similar values of gas exchange in this species under field conditions). Gas exchange in P. nigra also tended to decrease but less markedly. This species showed higher gs than P. halepensis in June, but no significant differences in July. A strong reduction of gs under drought conditions slows transpiration allowing plants to keep high water potentials. Consistent with this idea and our second hypothesis, P. sylvestris and P. nigra showed higher water-potential values than P. halepensis, both in our study (table 1) and in others studies (Lebourgeois et al 1998, Oliet et al 2002, Poyatos et al 2013, see also Matías et al 2017 for the simultaneous comparison of the three species). All these results imply therefore greater stomatal closure in P. sylvestris and P. nigra than in P. halepensis as dry conditions progress, leading to a strong reduction of gs that minimizes the falling of water potential, but seemingly at the expense of hindering photosynthesis. The limitation of growth imposed by stomatal closure is further supported by the lower growth exhibited by P. sylvestris and P. nigra relative to P. halepensis.
The results at the leaf level are mostly consistent with the measurements at the whole-plant level. The threshold for gs reduction with increase in AED was highest in P. halepensis (P < 0.003 in all cases; table 3; figures 4(d)-(f)). Moreover, in P. nigra and P. sylvestris transpiration did not increase as fast with AED as in P. halepensis (table 3). This further supports our first hypothesis and indicates that P. nigra and P. sylvestris had stronger stomatal control to air evaporative demand, which could be regarded as a strategy to limit water loss. According to these results, we should also expect a lower impact of air evaporative demand on photosynthesis in P. halepensis, but we did not find differences across species for this parameter (table 2). This could be due to the fact that the different environmental variables (e.g. PAR, VPD, temperature, etc.) affected assimilation in opposite directions throughout the day. Nonetheless, despite the higher transpiration rate and lower sensitivity of gs with increasing AED (figure 4), P. halepensis showed higher water-use efficiency (figures 3(c) and (d)), supporting the notion that the assimilation was less limited by climatic conditions in this species. Note that the values of iWUE at the whole-plant level contrast with those at the leaf level (particularly for the case of P. sylvestris), corroborating the relevance of measuring gas exchange in the whole plant in order to understand ecophysiological responses to evaporative demand. P. nigra and P. sylvestris also showed higher mortality, which could have been caused by carbon starvation. The lower growth and the positive correlation between growth of the first year and final survival observed in P. nigra and P. sylvestris could be an indication of carbon limitation (Pedersen 1998, Ogle et al 2000). However, this cannot be confirmed without evidence of a differential impact of drought on the non-structural carbohydrate concentration (Adams et al 2013, Maguire and Kobe 2015).
Figure 4. Within species relationship between transpiration rate (E), stomatal conductance (gs) and water-use efficiency (WUE) with air evaporative demand (PC1 from PCA analysis). Individuals are represented as different symbols. Shaded areas represent the noise in predictions due to variability between individual saplings of the same species.
Download figure:
Standard image High-resolution imageThe performance of P. halepensis was, therefore, much higher inasmuch as this species exhibited less stomatal control, but higher photosynthesis and water-use efficiency at the whole-plant level (figures 3(a), (c) and (d)). The strategy of P. halepensis to cope with drought consisted in maintaining open stomata and letting the water potential drop, allowing for a soil-root-leaf-atmosphere water continuum that can sustain higher gas-exchange capacity (figures 3(a) and (b)). In fact, the stem-water potentials in P. halepensis may reach values considerably lower than those reported in this study (up to −4.1 MPa, which is the lowest value for the genus; Oliet et al 2002, Choat et al 2012). By contrast, the three studied species show little differences in vulnerability to water stress-induced embolism, exhibiting similar values of 50 (water potential at which 50% of conductivity is lost, that are −2.80, −3.11, and −3.61 MPa for P. nigra, P. halepensis and P. sylvestris, respectively; Choat et al 2012, see also Delzon et al 2010). As a consequence, P. halepensis has a narrower safety margin (i.e. difference between the water potential at which significant hydraulic conductivity is lost and the water potential at stomatal closure; Klein et al 2011) than the other species, which might increase the risk of embolism. However, P. halepensis may also avoid embolism by a rapid stomatal response but at lower water potentials (Klein et al 2011). Thus, the differences in stomatal control are likely the main driver of the differential response to aridity, independently of xylem embolism resistance. In summary, our data suggest that a higher stomatal aperture under dry conditions enables P. halepensis to outperform P. nigra and P. sylvestris under water stress, despite that this implies higher water consumption (figure 3(b)). Although counterintuitive in terms of water economy, this strategy allowed P. halepensis to have higher WUE than the other, more conservative species. This maximizes plant growth, creating a positive feedback where increased growth (and very likely root growth in particular) will allow the plant to take up more water, sustaining the outperformance of this species with respect to the more conservative ones. We should note that, in contrast with our results, Manzanera et al (2016) reported higher transpiration and stomatal conductance in P. sylvestris than P. halepensis. This inconsistency could be due to the level of measurement (leaf vs. whole-plant level; Escalona et al 2003, Medrano et al 2012, 2015), which again supports the advantage of combining both approaches.
Plants may show changes in functional traits through ontogeny (Mediavilla and Escudero 2003b, Zhou et al 2015) and it is possible that differences between studies can be attributed in part to differences in ontogenetic stage of studied species (but see Cornelissen et al (2003) for consistency in functional traits across ontogeny). Therefore, extrapolation to adults of physiological results found in juveniles must be done carefully. However, it has been shown that adult trees of P. halepensis also appear to maximize carbon uptake by means of a narrow safety margin, as evidenced by the low levels of leaf water potential reported by Atzmon et al (2004). In addition, the pattern of water potential regulation between the three species is similar at the adult stage (Choat et al 2012). Finally, water potential at stomatal closure observed in adults of P. sylvestris (−1.9 MPa; Poyatos et al 2008) is congruent with the midday water potential (−1.84 MPa; table 1) and the lower gas exchange exhibited by this species in our experiment. Therefore, it is likely that differences in gas exchange between the saplings of the three studied species can be extrapolated to the adult stage.
As hypothesized, air evaporative demand was likely a driver explaining the contrasting responses observed among species. In this sense, P. nigra and P. sylvestris responded with stomatal closure to rising values of AED, whereas P. halepensis increased stomatal aperture (figures 4(d)–(f)). Note that the negative impact of air evaporative demand on stomatal conductance in P. nigra and P. sylvestris occurred despite the fact that the soil-water content remained in all cases above the wilting point (appendix S3). This is consistent with the fact that evaporative demand increases exponentially with temperature (Breshears et al 2013). Therefore, a high-soil water content might not be enough to offset the stress caused by rising temperatures under climate change, exacerbating in this way the effect of drought (Breshears et al 2013, Williams et al 2013). Our results therefore support the contention that these three species will face increasing evaporative demand in contrasting ways. The more conservative species, such as P. nigra, and mainly P. sylvestris, may show poorer performance and greater mortality (maybe by carbon starvation). By contrast, the Mediterranean P. halepensis may cope with much higher water stress than the other species because it can maintain CO2 uptake and photosynthesis by means of lower stomatal control. It should be borne in mind that the common garden experiment was conducted in a locality that is within the range of P. halepensis but out of the present range of the other species. However, climatic projections by the end of the 21st century show an increase in summer temperature (≥4 °C), and a reduction in rainfall (≥30%) for southern Europe (A2 scenario; Giorgi and Lionello 2008, IPCC 2013) that match the climatic conditions at the study site. Therefore, most of the area currently covered by P. nigra and large areas of the southern distribution of P. sylvestris will very likely undergo an increase in evaporative demand that might reduce their survival and performance. This could result in episodes of forest die-back as has already been reported in the last decade for these species under dry years (Sánchez-Salguero et al 2012). As a consequence, P. nigra and P. sylvestris may undergo a reduction of their distribution areas, while P. halepensis could colonize new areas at higher latitudes and elevations throughout Europe (García-Valdés et al 2013, 2015).
Conclusions
This study demonstrates that, under increased evaporative demand, P. nigra and P. sylvestris show a conservative strategy to avoid low water potentials even under high soil-water content, in contrast to P. halepensis, which maximizes CO2 uptake by means of weak stomatal control. These contrasting strategies could have consequences in performance, mortality, and finally the distribution of these species. Moreover, these results highlight the benefits of combining measurements at the leaf and whole-plant level in elucidating the response of species under a hotter drought scenario.
Acknowledgments
This work was supported by the projects ECOLPIN (AGL2011–24296) and Remedinal 3 (S2013/ MAE- 2719) of the Madrid Government, by a FPU fellowship from the Spanish Ministry of Education, Culture and Sport (FPU13/03410) to DS and by EU Marie Curie (FP7–2013-IOF-625988) fellowship to EPSC. We thank David Nesbitt for linguistic advice. We are also grateful to the Centro Nacional de Recursos Genéticos Forestales 'El Serranillo' (MAGRAMA) for cultivating the plants during nursery stage.