Abstract
This study presents the polymer coating onto metal-based structures by the thermal spray process. The deposition of polymers onto metal substrate has been developed by controlling the temperature in conventional thermal spray process. Properties of final polymer coating are investigated, such as microstructural, crystallization, mechanical, friction and corrosion properties. The objective of this is to deposit a polymer coating by controlling temperature in conventional thermal spray process and evaluate the coating properties for specific applications such as polymer coating on vacuum pump screws as abrasion resistant coating and on Kevlar fabric to provide strength for bullet proof soft armor vest for defense application. This study reports that as-sprayed coating can be used without post processing like annealing and filing. As-sprayed PEEK coating has a considerable hardness and adhesion strength found to be 17 ± 5 HV, 16 ± 3.0 MPa, respectively. However, post annealing treatment may introduce further improvements in wear and friction resistant properties of the final polymer coating system. The microhardness value has increased from 17 HV to 29 HV in average. The annealed semi-crystalline coatings exhibit the slightly lower friction coefficient than amorphous coating (as-sprayed); 0.31 in average versus 0.36. The wear rate of annealed coating is lower than the as-sprayed coating, decreased by approx 50% (10.5 in average versus 20.5 × 10− 6 mm3 N−1 m−1). The PEEK coating protects the base metal against corrosion and life against corrosion is found to be 25.4 μm.y−1; 81.75% efficiency in reduction of corrosion with respect to steel exposed in 5% NaCl solution.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
A number of industrial parts and structures are being coated with a layer of polymer coating. This coating protects the metal surface from humidity, corrosion, prevents excessive wear and serves as decorative purposes [1]. Poly-ether-ether-ketone (PEEK) Ultra-high-molecular-weight polyethylene (UHMW) and Linear low-density polyethylene (LLDPE) have become the most promising polymer materials and been more and more used in industry due to its excellent thermal stability, anti-corrosion, friction reduction and wear resistance [2–4]. PEEK and LLDPE polymers are used in hip, knee, and for spine implants [5, 6].
Thermally applied polymer powder coatings have been used for many years in commercial fluidized beds or with electrostatic spray guns. In appropriate for field use, these polymer application methods have been largely limited to job shop use. There are a few well established traditional coating processes are being used for deposit polymer coating, such as solvent-based system; powder coating process; fluidized bed coating process and flame spray coating process. Many polymer-based coatings are deposited by spray/brushing/dipping following by curing method for anti-corrosion, anti-graffiti, transparent hydrophobic, lubricating applications [7–13]. A recent study reports Polytetrafluoroethylene coating deposited by magnetron sputtering may act as oil adsorbing layer [14]. Many parts are made of polymer and plastics and they need to wear resistant in their working environment. A thin and hard ceramic coating of SiO2 by evaporation under vacuum is applied on top of a standard ophthalmic structure [15]. A few studies have reported superhydrophobic/icephobic surface was prepared by plasma treatment followed by plasma polymerization [16, 17]. However, cold spray method has been used to deposit antifouling (AF) protection. Copper particles embedded into polymer surfaces by cold spray (CS) have been confirmed as a method for antifouling (AF) protection [18]. A tin and aluminum coatings were deposited on polymer substrates using a low pressure cold spray [19].
Flame spray process allows formulated particles to be directed into a spray gun which incorporates oxygen-fuel combustion process, directed as a flame towards the substrate. Heat is transferred from the combustion to powder particles. Powder particles become molten and propelled towards substrate and create a uniform coating. This thermal spray process has added advantages over the other well established traditional coating processes viz. solvent-based system; powder coating process; fluidized bed coating process. There are some disadvantages of this process. (1) Flame combustion reactions are free radical and uncontrollable, that means combustion reaction is not limited to the fuel used. Free radical reactions always present in the flame combustion area cause addition and elimination reactions to occur, causing chemical changes in the polymer powder and final coating properties may get changed; (2) the polymer particles also may be oxidized by the hot uncontrolled flame temperatures; (3) In the same way, substrate may also get oxidized such as cloth, composites or even metal. Hence, the final coating starts to lose its properties.
Thermal sprayed polymer coatings on metal substrate using thermal spray method are not much studied and investigated. Only a few literatures are available. Recent investigations on polymer based thermal spray coatings on metallic substrates evince their contribution for friction reduction, anti-corrosion, bio-medical implant and anti-wear applications to accomplish the current industry demands [5, 6, 20, 21]. The spraying possibility of polymeric coating using different thermal spray processes and thereafter coating structural and mechanical characterization were substantially investigated in the earlier works [22–24]. Efforts are being made to identify new industrial applications of polymer coatings. For new industry applications, a polymer coating of PEEK is also applied on the industrial vacuum pump system parts such as screws. Graphene-based polymer composite coating onto the special fabric is being investigated for bullet proof soft armor vest for defense application. Both applications are being developed by thermal spraying method.
The deposition of polymeric materials through thermal spraying onto metal surface required optimal heat supply by specific nozzle design to flow polymer powder, proper substrate preparation and optimized spraying parameters. In this study an attempt has been made to solve existing problems in traditional flame spray process by employing a new flame spray nozzle. One-step coating process to deposit dense polymer coatings is presented. The objective of this work was to deposit the homogenous polymer (PEEK) coating by means of thermal spraying i.e. flame spraying onto a low carbon steel substrate and further improving coating's mechanical and tribological performance.
Experimental procedure
Materials and methods
PEEK polymer powder (Vitrex sale Ltd Lancashire, UK.) having a mean diameter of 25 μm is used as coating materials. PEEK has a glass transition temperature (Tg) of 143 °C and a melting temperature (Tm) of 343 °C. Low carbon steel plates with size of 80 × 50 × 3 mm, polymer rods having diameter of 1 inch were used as substrates material.
The PEEK coatings are deposited by newly developed thermal spray hardware at R&D facility, M/s Metallizing Equipment Company Pvt. Ltd Jodhpur, India. Flame spraying is known for the low powder particle velocity and low flame temperature, which is need to control, depends upon the polymer material's thermal characteristics and desired coating properties. To achieve a uniform coating thickness and consistent coating properties the coating process was carried out in a thermal spray booth using a Kuka robot. Prior to spraying, metallic substrates samples were properly cleaned in ultra sonicator with acetone for 5 min and grit blasted in order to increase the surface roughness, so that it could improve the adhesion strength of the coating with the substrate. A preheating pass was given to the substrate prior to deposition of the coating. Preheating of job is necessary step to be adopted in thermal sprayed polymer coating. The coating was cooled using compressed air after each pass of gun [20].
Coating characterizations and testing
As-sprayed coatings were tested and characterized in the R&D laboratory of MEC India. Cross-sections of the samples were examined under the Scanning Electron Microscope (Carl ZEISS Evo18, UK) equipped with Backscatter electron detector (BSC) and EDS analysis (Oxford Instruments, United Kingdom). Software 'Image-J' was used to distract data on area percentage porosity, pore and interconnections sizes from BSE images. The microhardness was examined with a SHIMADZU HMV-G-21ST, Japan) automatic microhardness tester as per ASTM-E384 under a load of 1 N and a dwell time of 15 s and fifteen measurements were taken on the coated sample. The tensile strength of the coating was tested using INSTRON Digital Tensile Testing Machine (Model: 5969 USA) according to ASTM-C633. The adhesion strength of the coating was tested by pull-off strength of coatings using portable adhesion test as per ASTM D 4541. Potentiodynamic polarization studies of base metal and PEEK coated samples were conducted by Gamry instrument Reference 600+ Potentiostat/Galvanostat/ZRA to determine the corrosion potential, corrosion current and percentage protection efficiency in corrosion solution containing 53 g NaCl in one-liter distilled water. The scan rate was 1 mV s−1 with respect to reference electrode.
Samples were annealed at 150 °C, 200 °C, 250 °C, 300 °C for only 15 min. In addition, two samples were also treated at 250 °C for 1 and 5 min, respectively. The annealing treatment was similar for DSC, wide angle XRD (WAXD) and friction testing samples. The iced water used to immersion of annealing samples. TA instruments make DSC chamber (2010 DSC, Newscastle, DE) was used to measure thermal properties of as sprayed and annealed condition coating samples. The measurement was taken out with nitrogen gas flow atmosphere at 20 cm3 min−1. Thermal events i.e. endothermic or exothermic was recorded in a specific temperature scan range of 20° to 380 °C. A steady state heating control rate 10 °C min−1 was given to plot relative heat flow as a function of increasing temperature. WAXD measurement to find out the coating crystallinities and the differences in the crystal perfection was conducted using Simens D5000 automatic diffractometer with cobalt anticathode (λ = 1.79 Å). A WAXD pattern was obtained for a 2θ range of 10° to 40° with scanning rate of 5° min−1.
Sliding wear test was performed using a ball on disc setup. Spherical 100Cr6 bearing steel ball of 12.5 mm diameter and surface roughness 0.02 microns was used as a counterpace material against the coating surface. The hardness of the 100Cr6 steel ball was 62.5 HRC. Sliding wear testing of PEEK coated samples were conducted with a Taylor-Hobson Surtronic 3P profilometer. Wear rate data was obtained using volume loss method for a fixed sliding distance of 500 m average of 3 samples for each conditions. The friction and wear test parameters are given in table 1. Friction force data was continuously recorded during the testing of each sample.
Table 1. Friction and wear test parameters.
| Parameter | Value |
|---|---|
| Load | 15 N |
| Sliding speed | 200 mm s−1 |
| Track diameter | 3 cm |
| Temperature | 25 °C |
| Humidity | 60%–65% |
Results and discussion
Coating powder and as-sprayed coating microstructure
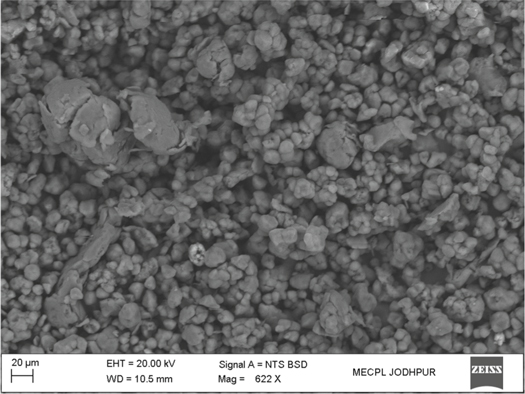
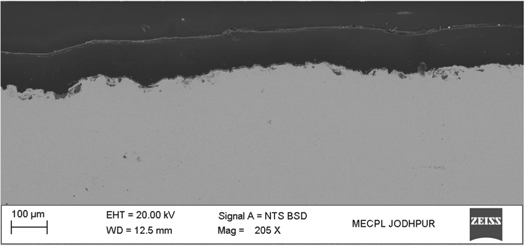
It is very difficult to deposit a polymer coating by thermal spray process due to higher temperature of the flame produces in thermal spraying. At high temperature polymer powder burns in flame during the deposition and it turns into carbon. Also, overheating cause to polymer oxidation and chain scission, thus leading to degradation of coating properties. Addressing this problem, a new thermal spray apparatus is designed and developed in MECPL R&D laboratory by employing a new spray nozzle. The spray nozzle has a unique design which reduces flame temperature near about melting point of a polymer powder. After successful development of the spray nozzle, at first controlled spray parameters were optimized for a dense PEEK coating. The morphology of PEEK powder used in present work is shown in figure 1. Powder particles were having irregular shaped morphology. Microstructures of as-sprayed PEEK coating is shown in figure 2. It can be seen that as-sprayed PEEK coating is very dense and crack free. Moreover, coating surface is smooth and shows good interlocking with metallic substrate. It can be inferred from cross-sectioned SEM micrograph that the flame heat was enough for sufficient melting of PEEK powder and resulted into the completely coalesced with the substrate material. The coating thickness is 100 μm.
Figure 1. Morphology of PEEK powder.
Download figure:
Standard image High-resolution imageFigure 2. SEM micrographs as-sprayed PEEK coating.
Download figure:
Standard image High-resolution imageDSC analysis
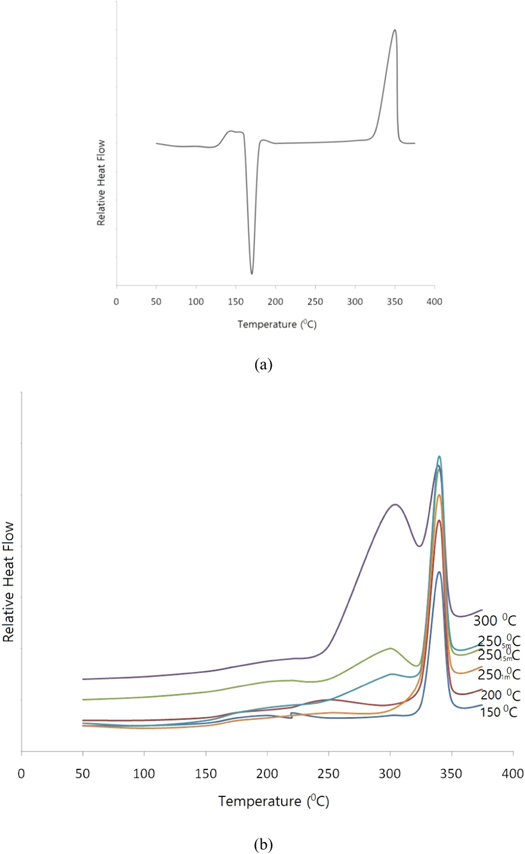
Figure 3(a) shows the DSC thermogram corresponding to the as-sprayed coating. Exothermic peak is found at 143 °C which means crystallization. Thus, as-sprayed coating has an amorphous structure. The coating's degree of crystallinity represented by the area under the melting peak of DSC curve and peak width represents the melting temperature range.
Figure 3. DSC thermograms of (a) as-sprayed coating; (b) annealed samples.
Download figure:
Standard image High-resolution imageFigure 3(b) shows the DSC thermo grams of Samples were annealed at 150 °C, 200 °C, 250 °C, 300 °C for 15 min. In addition two samples were treated at 250 °C for 1 and 5 min respectively. As shown in figure 3(b), no crystallization exothermal peak was present above the glass transition temperature. This revealed that annealed coating samples exhibit a semi-crystalline structure.
It is found that a minor endothermic peak can be noted in each sample. This endothermic peak appears at near above the decided annealing temperature. The coating's annealing conditions affects the presence of minor peak while the main peak is independent on the annealing condition. A deviation in minor endothermic peak position was observed towards the decided annealing temperature. This shift of minor endothermic peak shows metastable/non-equilibrium state of melting [25].
A number of interpretations are available to explaining endothermic double melting of annealed polymers. But their evaluation depends upon the thickness of lamellar or crystal structure [26–30]. Cebe and Hong model [27] based on dual lamellar thickness considered to be more agreeable as supported by a few investigations [25, 31, 32]. This model [27] defines the presence of thin lamellar between the thick lamellar of separate stacks.
There are types of crystallization. In the beginning of the annealing, thick lamellae egress from the amorphous matrix and in-between these lamellar structures stay amorphous. Later, small and thinner lamellae egress in these amorphous regions. The coexistence of these dual lamellae in different stacks could explain the double melting behaviour in annealed samples.
Thick lamellae melting are shown by the main peak on DSC scanning. The peak occurrence at low temperature represents the thinner lamellae melting within semi-crystalline structure. From the DSC analysis of samples annealed 250 °C for 1 and 5 min, respectively, the absence of crystallization exotherm in 1 min annealed sample indicates that the first crystallization step for forming thick lamellar structure is completed after 1 min of annealing treatment at 250 °C. A shift in minor peak position towards higher temperature and greater enthalpy noted with increased holding time (5 min).
Quantification and analyses of coating crystallinity
Wide-Angle x-ray Diffraction (WAXD) analysis was done of as-sprayed and annealed samples treated at different temperatures. The results show the influence of the annealing temperature and holding time on the of PEEK coatings. With increasing the annealing temperature and holding time, the diffraction intensity is increases and it means increased crystallinity. The coating crystallinities values are listed in table 2.
Table 2. Crystallinities in as-sprayed and annealed PEEK coatings.
| Coatings | As-sprayed | 150 °C for 15 min | 200 °C for 15 min | 250 °C for 15 min | 300 °C for 15 min | 250 °C for 1 min | 250 °C for 5 min |
|---|---|---|---|---|---|---|---|
| Crystallinity | 0 | 7.2 | 22.4 | 26.7 | 28.5 | 22.3 | 24.6 |
Mechanical properties
The microhardness measurement was carried out on polished cross-section of the as-sprayed coatings. The average of 15 identical hardness readings is taken along the cross-sections of each sample. It was observed that as-sprayed PEEK coating has a considerable hardness and found to be 17 ± 5 HV. Adhesion strength is an important factor in thermal spray coatings because it is directly related to the performance and durability of the coating as it directly influences the fatigue life of the coating. In addition tensile strength of the coatings was also investigated by the tensile test using INSTRON Digital Tensile Bond Testing Machine (UTM, Model: 5969, USA) and portable Pull-Off strength adhesion test (PosiTest® Pull-Off adhesion Tester). From these both tests the adhesion strength of the as-sprayed PEEK coatings was found to be 15 ± 5.0 MPa, and 16 ± 3.0 MPa respectively.
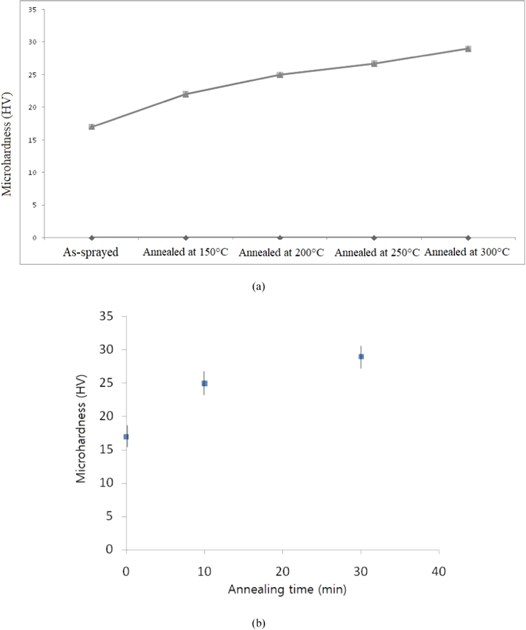
Figures 4(a) and (b) show the effect of annealing temperature and it's holding time on the coating hardness. An improvement in hardness value was observed for annealed coatings in comparison to as-deposited PEEK coating. The microhardness value is increased from 17 HV to 29 HV in average. After comparing the DSC and WAXD scanning results, it can be noted that semi-crystalline coatings possessed higher hardness value than the amorphous one. The annealing temperature and holding time have a direct influence on the coating microhardness. With an increase in the annealing temperature from 150 to 300 °C with holding 1 to 15 min correspondingly, the microhardness of the annealed coatings increase from 22 to 29 (HV) as shown in figure 4.
Figure 4. (a) Influence of annealing temperature on the coating microhardness; (b) Influence of holding time on the coating microhardness.
Download figure:
Standard image High-resolution imageCoefficient of friction and wear rate
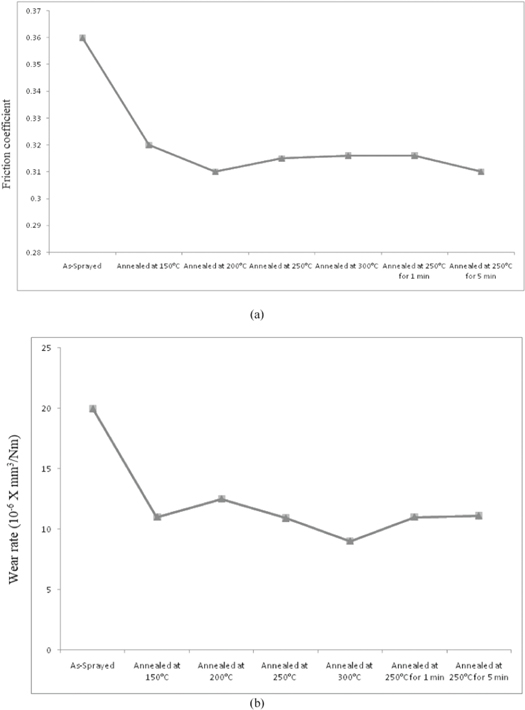
Figure 5(a) shows the mean values of the coefficient of friction of the PEEK coatings. Result shows that the annealed semi-crystalline coatings exhibit slightly lower friction coefficients than amorphous coating (as-sprayed); 0.31 in average versus 0.36. However, it can be observed that different annealing conditions (150 °C, 200 °C, 250 °C, 300 °C for 15 min; 250 °C for 1 and 5 min) do not have much influence on friction coefficient. Figure 5(b) shows the wear rates data of the coatings. The wear rate of annealed coating is lower than the as-sprayed coating, decreased by approx 50% (10.5 × 10−6 mm3 N−1 m−1 in average versus 20.5 × 10−6 mm3 N−1 m−1). The volume loss of Peek coatings at different conditions has listed in table 3. Different annealing conditions do not have much influence on the wear rate, which is similar with the friction coefficient. The better tribological performance of annealed coating is result of increased hardness and rigidity as crystalline coating exhibit higher hardness than the amorphous as-sprayed coating. It indicates that coating deformation is decreased under the load condition [20, 32].
Figure 5. (a) Mean friction coefficient; (b) wear rate.
Download figure:
Standard image High-resolution imageTable 3. Volume loss of PEEK coating at different conditions.
| Coatings | As-sprayed | 150 °C for 15 min | 200 °C for 15 min | 250 °C for 15 min | 300 °C for 15 min | 250 °C for 1 min | 250 °C for 5 min |
|---|---|---|---|---|---|---|---|
| Volume loss (mm3) | 0.1482 | 0.0819 | 0.0934 | 0.081 | 0.0678 | 0.080 | 0.083 |
Worn surface analyses
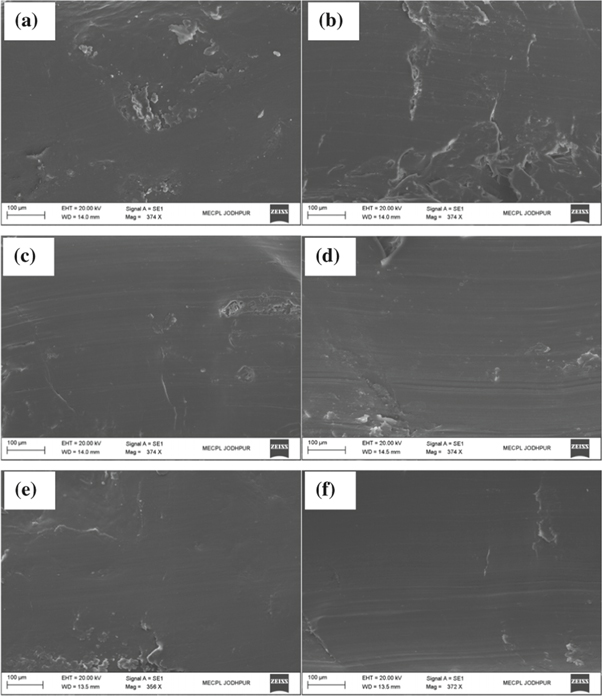
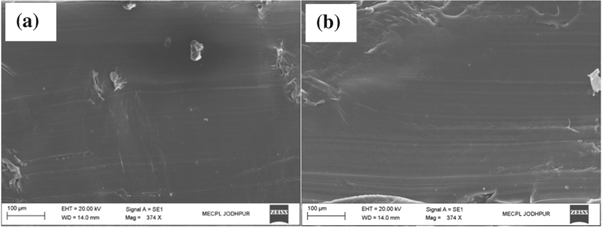
Figure 6 shows the SEM micrographs of worn surfaces of PEEK coating under as-sprayed and annealed conditions. Worn surface analyses of PEEK coating revealed that main material removal mechanisms were plastic deformation and ploughing. In as-sprayed coating, removal of PEEK coating by ploughing, cutting and coating surface exfoliation was observed as shown in figure 6(a). Fatigue tearing and a displacement of splat were also observed as shown in figure 6(b). The coating with high wear rate showed deeper and yawn ploughing marks (figures 6(a), (b)). In as sprayed coatings, more wear debris formation during sliding process attributed to higher friction coefficient compared to annealed coating samples. Improved mechanical and structural properties of the PEEK coating with different annealing conditions are supported with worn surface wear mechanisms. After annealing of PEEK coating, a less damage of worn surfaces was observed as shown in figures 6(c)–(f), respective of annealed temperature. Annealed at 250 °C for 1 min didn't showed any significant improvement in wear mechanisms. Worn surface showing severe cutting and loose wear particles presents mild abarsion wear mechanisms, as shown in figure 7(a). While coating annealed at 250 °C for 5 min (figure 7(b)) was comparaitively better in term of wear resrstance and wear mechanisms i.e. platic deformation. Coating annealed at 300 °C offered higher shear resistance asresulted into decrease intensity of plastic deformation and no coating surface exfoliation [20]. Viscoelasticity of polymer coating could also be affected the tribological behavior of polymer coating [33, 34]. On the other hand, improved coating hardness at 300 °C anneled temperature could be the another reason for better wear behaviour of PEEK coating. Moreover, the rigidity is increased when crystallization occurs in polymer from an amorphous structure [35].
Figure 6. SEM micrographs of worn surfaces at different conditions; (a, b) As sprayed (c) Annealed at 150 °C (d) Annealed at 200 °C (e) Annealed at 250 °C (f) Annealed at 300 °C.
Download figure:
Standard image High-resolution imageFigure 7. SEM micrographs of worn surfaces at (a) Annealed at 250 °C for 1 min (b) Annealed at 250 °C for 5 min.
Download figure:
Standard image High-resolution imagePolarization studies
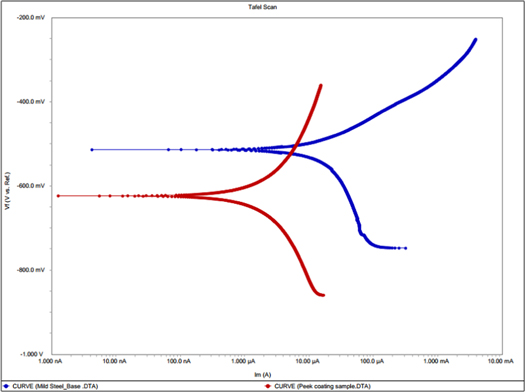
The polarization curves of base metal and as-sprayed PEEK coated samples are shown in figure 8. Thecathodic curve plots shows that PEEK as well as steel sample exhibited oxygen reduction reaction but the cathodic current density is significantly reduced when PEEK coating was applied on steel substrate which reduces the penetration of NaCl solution owing to the barrier type of protection. As the applied potential was increased, from the corrosion potential (Ecorr), the anodic current density increases. The anodic current density of steel sample increased suddenly after Ecorr which infer that steel substrate causes pitting corrosion rather than uniform owing to the aggressiveness of NaCl which attack locally at steel substrate. On the other hand, the increment in anodic current density of PEEK sample gradually increased which suggested that polymeric coating inhibited the attack of Cl- ion on steel substrate. The anodic current density of PEEK applied coating is greatly reduced compare the steel sample which attributed to the barrier protection. PEEK coating is uniform and contains negligible porosity (figure 2) which does not allow to ingress of NaCl solution. Although, 5% NaCl is very high amount where PEEK coating sustain significantly in regards of corrosion resistance while steel possesses very high anodic and cathodic current density.
Figure 8. Polarization curves of As-sprayed PEEK coating compared with base metal.
Download figure:
Standard image High-resolution imageThe electrochemical parameters such as corrosion current (Icorr) and corrosion potential (Ecorr) were extracted after fitting of polarization curve in Tafels regions and results are show in table 4. The corrosion rates (μm.y−1) were calculated as [36]:
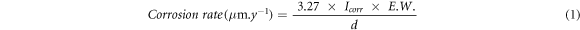
where Icorr, E.W. and d represent corrosion current density (μA cm−2) obtained from dividing the total surface area of the working electrode by the corrosion current (μA), the equivalent weight (g mol−1), and the density (g cm−3) of the substrate, respectively.
Table 4. Electrochemical analysis data of base metal and as-sprayed PEEK coating.
| Base metal | PEEK coating | |
|---|---|---|
| Parameter | value | value |
| Icorr | 12.00 μA cm−2 | 2.190 μA cm−2 |
| Ecorr | −514.0 mV | −624.0 mV |
| Corrosion rate | 139.50 μm.y−1 | 25.4 μm.y−1 |
The corrosion potential (Ecorr) for base metal and PEEK coatings is found to be −514.0 mV and −624.0 mV, respectively. The active Ecorr of PEEK owing to the polymeric coating which shows activeness. However, the Icorr of the steel and PEEK is found to be 12 μA cm−2 and 2.19 μA cm−2, respectively. The Icorr of the PEEK sample is reduced by around 5.5 times compare to steel sample. The corrosion rate of steel and PEEK sample is found to be 139.50 μm.y−1 and 25.4 μm.y−1, respectively. This result suggests that after using PEEK coating, the corrosion rate of samples is reduced significantly. Thus, it is suggested that PEEK could be one promising coating to protect the steel substrate from corrosion in aggressive environment.
The efficiency (%) of the coating was calculated as:
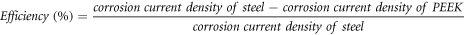
Thus, it is found that PEEK shows 81.75% efficiency in reduction of corrosion with respect to steel exposed in 5% NaCl solution.
Conclusions
Dense PEEK coatings are fabricated by means of thermal spray process. This study reports novel results of depositing PEEK coating for future and existing industrial applications. As-sprayed PEEK coating exhibit a dense microstructure, good interface and bonding and mechanical properties. As-sprayed PEEK coating has a considerable hardness and adhesion strength found to be 17 ± 5 HV, 16 ± 3.0 MPa, respectively. Post annealing treatment introduced a crystallization occur in the coating and resulting enhancement in mechanical properties of the PEEK coating. The microhardness value has increased from 17 HV to 29 HV in average. Furthermore, the PEEK coatings exhibit lower friction coefficients and wear rates after annealing treatment; 0.31 in average versus 0.36. The wear rate of annealed coating is lower than the as-sprayed coating, decreased by approx 50% (10.5 in average versus 20.5 × 10−6 mm3 N−1 m−1). Higher corrosion rate was observed for base metal (139.50 μm.y−1), whereas, PEEK coating protects the base metal against corrosion and life against corrosion is found to be 25.4 μm.y−1. The PEEK coating shows 81.75% efficiency in reduction of corrosion with respect to steel exposed in 5.3% NaCl solution. Such properties are can be suitable for many industrial applications such as for screws for vacuum pump and system, anti-corrosion and anti-chemical applications, orthopaedic implants, polymer coatings on fabric for bullet proof barrier etc.