Abstract
The Direct Metal Laser Sintering (DMLS) process is widely used for biomedical applications and to fabricate Cellular Structures (CS). Titanium alloy (Ti64) CS were modelled as a honeycomb structure with variations in pore diameters (0.8 mm, 0.9 mm and 1.0 mm) and interpore distances (1.6 mm, 1.7 mm and 1.8 mm) in this research work. The maxillofacial region is considered for implementation of CS, with mandible being the selected application. Finite Element Analysis (FEA) was carried out on all CS models and the least von-mises stress was observed to be 48.67 MPa and the corresponding Young's modulus was calculated to be 34.76 GPa. Based on FEA results, CS were fabricated through DMLS and tested for compressive behaviour. The average Young's modulus was calculated to be 32.10 GPa, the average compressive strength was evaluated to be 51.25 MPa and the average strain energy was calculated to be 0.94 J, respectively. The FEA and experimental results were in correlation with each other. Since CS was able to withstand the required load for mandibular implant application, it can be considered as safe. The compressive behaviour of Ti64 CS was observed to be sensitive to varying rate of loading. A ductile fracture was observed upon fractography analysis and the CS exhibited a martensitic microstructure, which accounts for good compressive strength. The average surface roughness of the CS was measured to be 1.26 μm, which is within a suitable range of tissue growth for mandibular implant. The tissue culture was done to study the biocompatibility of CS and an increase in the percentage of cell viability was observed as 55% on day 1, 68% on day 3 and 75% on day 5, respectively.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Laser-based Additive Manufacturing (AM) processes result in the formation of α-martensite due to faster cooling, and these processes are characterized by high strength and low ductility. One such laser based process, namely DMLS, uses lasers to fuse metallic powders to create parts from a 3D computer model. DMLS is widely used for the fabrication of implants and CS [1–3]. CS are a class of advanced materials that are light and stiff, providing high moduli with low density. They are characterized by good strength and low weight. For the same application, CS helps in the reduction of material when compared to a solid structure by maintaining the required strength. Thus the material consumption is reduced i.e. the weight of the structure is reduced, leading to savings in cost. Pore size / diameter and interpore distance are important parameters for CS in determining their mechanical strength and biocompatibility. CS are widely used in various biomedical applications, especially in biological scaffolds [4–7]. Tissue engineering involves the regeneration of damaged tissues, where cells from the body are combined with porous biomaterials to guide the growth of new tissues and thus helping in new tissue formation [8, 9]. Implants and scaffolds are widely used for the regeneration and reconstruction of bone [10]. The maxillofacial region is considered in this work, while mandible is the selected application. The mandible is the lowermost bone in the maxillofacial region and on loading, gets deformed. During biting and mastication, mandible is subjected to compressive, tensile, shear and torsional stresses and strain patterns [11]. Out of these patterns, compressive stress is a major factor due to the biting force that acts on the mandible. Therefore analyzing compressive behaviour is of importance for mandibular implant [11, 12]. Titanium alloy (Ti64) is an ideal material for many engineering and medical applications, especially for the production of implants [13–15]. Therefore it is selected for this work. It is desirable to analyze numerically the mechanical behaviour of CS before fabricating them. FEA is a successful technique used for the same. Maximum distortion energy theory (σvms) is a failure criterion that uses von-mises stress (equation 1) to predict failure of the material, where σx, σy and σz are the directional stresses in the respective axis.
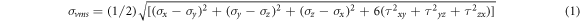
To determine the material yielding under any condition of loading, von-mises stress is beneficial and is thus utilized in this work to find the design with good compressive strength among the various combinations of pore diameters and interpore distances. Additive manufacturing is widely used for the fabrication of CS, scaffolds and complex structures that are difficult to be fabricated by conventional techniques [16, 17]. Therefore, in this study, the CS are fabricated using DMLS process. The compressive behaviour of titanium alloy (Ti64) CS is related to many parameters, out of which the pore shape, pore size and interpore distance are of great importance. The novelty of this research work is that the combined effect of pore sizes and interpore distances of Ti64 CS fabricated by DMLS for mandibular implant application are not reported in the literature so far, but the same is investigated here. Since hexagonal honeycomb structure is proven for its good mechanical properties, CS are modelled accordingly in this study. The modelled CS are supported by appropriate FEA simulations, experimentation and microstructural analysis. In addition to that, biocompatibility and surface roughness are studied to validate the CS.
2. Material and methods
2.1. Selection of material
EOS Titanium Ti64 was selected in this study based on its biocompatibility. Its material composition is shown in table 1 [18]. This titanium alloy has good mechanical properties, corrosion resistance and low specific weight. Spherical shaped titanium powder with particle size 30 to 60 μm was used, as shown in figure 1.
Table 1. Composition of EOS titanium Ti64 material.
| Material | Composition (%) | |
|---|---|---|
| Titanium | Balance | |
| Aluminium | 5.5–6.75 | |
| Vanadium | 3.5–4.5 | |
| Oxygen | <2000 ppm | |
| Nitrogen | <500 ppm | |
| Carbon | <800 ppm | |
| Hydrogen | <150 ppm | |
| Iron | <3000 ppm | |
Figure 1. Spherical shaped Ti64 powder at 500 X.
Download figure:
Standard image High-resolution imageThe powder composition was verified using Energy Dispersive x-Ray (EDX) analysis, as indicated in figure 2. The material properties are mentioned in table 2 [18].
Figure 2. EDX spectrum of Ti64 powder.
Download figure:
Standard image High-resolution imageTable 2. Material properties of Ti64.
| Property | Value (units) |
|---|---|
| Density | 4.41 g cm−3 |
| Young's Modulus | 110 GPa |
| Tensile Strength | 1290 MPa |
| Yield Strength | 1140 MPa |
| Poisson's Ratio | 0.31 |
2.2. Modelling of CS
Cellular structures of compression specimen based on ASTM E9 standard (12.7 mm × 12.7 mm × 25.4 mm) were modelled using solidworks software with various pore diameters and interpore distances. There was an increase in von-mises stress for the pore diameters below 0.8 mm. For pore diameters above 1 mm, there was a decrease in wall thickness, thus leading to a decrease in compressive strength. Therefore the pore diameters were selected as 0.8 mm, 0.9 mm and 1 mm, respectively. As per the manufacturing feasibility, the minimum wall thickness was maintained as 0.6 mm. Therefore, the interpore distances were selected as 1.6 mm, 1.7 mm and 1.8 mm, respectively. Figure 3(a) denotes CS parameters and figure 3(b) represents CS dimensions according to ASTM standard. The 3D model of CS is presented in figure 3(c). Based on the manufacturing feasibility, totally nine combinations of pore diameters and interpore distances were considered in this study to have good compressive strength and cell growth [18].
Figure 3. (a) Representation of CS parameters; (b) ASTM standard honeycomb CS; (c) 3D Model of CS.
Download figure:
Standard image High-resolution image2.3. Finite element analysis of CS
Material properties of EOS titanium Ti64, namely, Young's modulus, density and poisson's ratio, were given as input to FEA (table 2) and von-mises stress formation was observed, using abaqus software (figure 4).
Figure 4. Compression contour of the CS model with 1 mm pore diameter and 1.6 mm interpore distance.
Download figure:
Standard image High-resolution imageThe normal load acting together on molar, premolar and incisor teeth is 510 N and the permissible von-mises stress is 180 MPa [19]. Hence in this study, a compressive load of 510N was applied and the CS behaviour was studied. The CS model was fixed at the bottom using encastre boundary condition. Since the structure is porous, it was assembled beneath a rigid plate in order to ensure uniform application of load. The load was applied to the top of the rigid plate. Friction between the plate and structure was assumed as 0.1 and the tetrahedral mesh was used. Since the application involves a lower magnitude of load, a linear elastic analysis was performed [19]. For better understanding and comparison of CS models, stress-strain curves are discussed in respective sections.
The stress-strain curves for compressive behaviour of all nine CS models are shown in figures 5(a)–(c), respectively. A linear behaviour is observed for all the CS models. From these curves, Young's modulus of the CS models was calculated and is denoted in table 3. The von-mises stress values of nine combinations of CS models are represented in table 4.
Figure 5. Stress-strain curves of compressive behaviour for pore diameter (a) 0.8 mm (b) 0.9 mm (c) 1 mm.
Download figure:
Standard image High-resolution imageTable 3. Young's modulus of various CS models (GPa).
| Interpore distance (mm) | |||
|---|---|---|---|
| Pore diameter (mm) | 1.6 | 1.7 | 1.8 |
| 0.8 | 31.76 | 31.24 | 30.76 |
| 0.9 | 32.31 | 32.06 | 31.94 |
| 1.0 | 34.76 | 33.56 | 33.10 |
Table 4. Von-mises stress values of various CS models (N/mm2).
| Interpore distance (mm) | |||
|---|---|---|---|
| Pore diameter (mm) | 1.6 | 1.7 | 1.8 |
| 0.8 | 55.83 | 59.36 | 60.36 |
| 0.9 | 51.77 | 52.81 | 54.29 |
| 1.0 | 48.67 | 49.14 | 49.65 |
The relationship between pore diameter, interpore distance and von-mises stress is plotted in figure 6. From the results, it was observed that the CS model with 1 mm pore diameter and 1.6 mm interpore distance had minimum von-mises stress (48.67 MPa) compared to other CS models. The reason can be explained using the relationship between strain energy U (energy stored in the elastic body under loading), V (volume of the structure), σ (stress) and ε (strain), as given below;
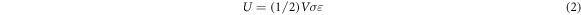
From equation 2, it can be noted that volume and strain energy are directly proportional. Therefore out of the nine models, this particular combination has the minimum volume that accounts for minimum strain energy. Strain energy has a linear relationship with von-mises stress and thus, the energy-absorbing behaviour of this particular combination of CS resulted in minimum von-mises stress formation. Also this particular CS model has the highest value of Young's modulus (34.76 MPa) among other models. Hence, this CS model was considered safer and desirable compared to other CS models and thus selected for fabrication.
Figure 6. Relationship between pore diameter, interpore distance and von-mises stress.
Download figure:
Standard image High-resolution image2.4. Fabrication of CS by DMLS
The CS after FEA were fabricated using EOSINT M 280 DMLS machine with 60 microns layer thickness and the support structures were placed at the base plate to support the parts at a 45 degree orientation. The CS was built layer-wise by sintering the powders using a 400 W Yb-fibre laser. Since components are subjected to residual thermal stresses in metal AM, as a stress-relieving mechanism, the samples have been subjected to heat treatment. In this work, the residual stresses were removed by subjecting the fabricated component to heat treatment for 8 hours at a temperature of 800 °C. The support structures were machined and thus removed. The surface quality was improved by means of the sandblasting technique. The fabricated specimen is shown in figure 7.
Figure 7. Fabricated Ti64 CS.
Download figure:
Standard image High-resolution image2.5. Dimensional accuracy of CS
DMLS process is used for fabricating complex structures and biomedical implants [2, 3]. The accuracy of any fabricated structure is important since it is aimed at a particular application. Out of that, the medical application requires utmost accuracy since it deals with human beings and other living things. Therefore, checking the dimensional accuracy of the fabricated component is important, even though DMLS is a suitable process for biomedical application. Hence, the pore diameter and wall thickness were measured using the Video Measuring System (VMS). Table 5 shows the measurements of pore diameter and wall thickness for the fabricated CS. The average value of pore diameter (1.044 mm) and wall thickness (0.614 mm) obtained is represented in figure 8. It can be noted that the dimensions of fabricated CS have only minor deviations with respect to the original dimensions of the CS model (1 mm pore size and 0.6 mm wall thickness). Therefore it was considered to be suitable for the proposed application of mandibular implant.
Table 5. Pore diameter and wall thickness of fabricated CS.
| Pore diameter (mm) | Wall thickness (mm) | |||||
|---|---|---|---|---|---|---|
| S. no. | Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 |
| 1 | 1.100 | 1.044 | 1.036 | 0.540 | 0.636 | 0.592 |
| 2 | 1.072 | 1.027 | 0.998 | 0.589 | 0.657 | 0.539 |
| 3 | 1.056 | 1.044 | 1.123 | 0.618 | 0.614 | 0.581 |
| 4 | 1.096 | 1.037 | 1.043 | 0.524 | 0.597 | 0.677 |
| 5 | 0.991 | 0.992 | 0.995 | 0.589 | 0.599 | 0.574 |
| Average | 1.044 | 0.614 | ||||
Figure 8. Average pore diameter and wall thickness of CS.
Download figure:
Standard image High-resolution image2.6. Compression testing
After fabrication and dimensional analysis, CS was tested for compressive behaviour. The sample was placed between the two fixtures, as shown in figure 9 and compression testing was performed on universal Testing Machine (UTM).
Figure 9. Sample under compressive load.
Download figure:
Standard image High-resolution imageA compressive load of 510 N was applied at the rate of 1 mm min−1 [19]. Three samples were tested in order to ensure the repeatability of results. The samples were able to withstand more than 510 N, which is the required load to withstand in the case of mandibular implants [19]. Figure 10 represents the stress-strain curves of the tested samples. A linear relationship was observed for all three samples. From these curves, Young's modulus was calculated. The experimental results obtained are tabulated in table 6. The average Young's modulus was calculated to be 32.10 GPa, the average compressive strength was evaluated to be 51.25 MPa and the average strain energy was calculated to be 0.94 J, respectively.
Figure 10. Stress-strain curves of tested samples.
Download figure:
Standard image High-resolution imageTable 6. Experimental results of compression test.
| Sample no. | Young's modulus (GPa) | Compressive strength (MPa) | Strain energy (J) |
|---|---|---|---|
| Sample 1 | 30.20 | 49.16 | 0.91 |
| Sample 2 | 33.80 | 53.40 | 0.98 |
| Sample 3 | 32.30 | 51.20 | 0.94 |
2.7. Cell culture
Even though Ti64 material is biocompatible, the cell culture of fabricated CS (with 1 mm pore diameter and 1.6 mm interpore distance) is carried out to determine the percentage of cell viability. The study was carried out using nine samples fabricated by DMLS with the dimensions of 10 mm × 10 mm × 1 mm. Figure 11 shows the samples placed for cell culture into 24-well plates. To mimic the mandibular bone cells, MG-63 osteosarcoma cells were used [20]. The cell growth was studied in three different time periods (day 1, day 3 and day 5) using three samples for each period to ensure the repeatability of results.
Figure 11. Samples placed for cell culture in 24-well plates.
Download figure:
Standard image High-resolution imageA flask with a surface area of 25 cm2 was used and it contained Dulbecco's Modified Eagle's Medium (DMEM). This medium was supplemented with 1.5 g l−1 sodium bicarbonate, 10 mg ml−1 streptomycin, 10% fetal bovine serum, 10,000 units ml−1 penicillin and 25 μg ml−1 amphotericin B. Assessment of cell viability was carried out using the standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [20, 21].
3. Results and discussion
3.1. Comparison of FEA and experimental results
From the FEA results of CS as discussed in section 2.3, all nine models resulted in von-mises stress less than the limit value of 180 MPa. The CS with 1 mm pore diameter and 1.6 mm interpore distance had the highest compressive strength and was fabricated using DMLS. Hence the stress-strain curve of this CS model was selected for comparison with experimental results. The stress-strain curve of sample 3 was selected from the experimental results since it nearly represents the average of experimental values.
Figure 12 shows the comparison of experimental and FEA results. From the figure, it is observed that the stress-strain curves of compressive behaviour obtained through experiment and FEA follow a linear pattern. In both the cases, the strain of the object is directly proportional to the applied stress within the elastic limit, according to modern theory of elasticity based on generalization of Hooke's law. Thus, the FEA and experimental results are found to be in correlation with each other.
Figure 12. Comparison of experimental and FEA results for compressive behaviour.
Download figure:
Standard image High-resolution image3.2. Strain rate sensitivity of CS
The compressive behaviour of CS was studied under various loading rates, namely 1 mm min−1, 2 mm min−1, 3 mm min−1, 4 mm min−1 and 5 mm min−1, respectively. The tests were conducted at room temperature [22]. Based on incremental changes in strain rate, corresponding changes are observed in stress values [23]. The compressive behaviour of Ti64 CS is observed to be sensitive to varying rate of loading. The strain rate sensitivity is represented in figure 13. A linear trend is observed and there is a variation in stress-strain curves for different loading rates [22, 23]. There is a shift in curve towards right when the loading rate is increased from 1 mm min−1 to 2 mm min−1, thus decreasing the stress value for the same strain percentage. Similar trend is observed for 3 mm min−1, 4 mm min−1 and 5 mm min−1 rate of loading. The least value of stress for the same strain percentage is observed at 5 mm min−1 rate of loading. Thus, the stress-strain characteristics of the fabricated CS are dependent on the rate of loading.
Figure 13. Strain rate sensitivity of the CS.
Download figure:
Standard image High-resolution image3.3. Fractograph analysis
The Ti64 CS were printed at 45° orientation as mentioned in section 2.4. To reduce the laser exposure to the surface area of the part (thus reducing the thermal stresses developed during fabrication under the metal using AM technique) and also to have an optimum printing time, this particular orientation was followed. Figure 14(a) shows the fabricated sample at a 45° orientation. The samples were subjected to compressive load until fracture to study its fractography [24]. Three samples were tested in order to ensure the repeatability. The average break load observed during the fracture is 44.2 kN. The crack is propagated at an average angle of 46.4° in the three CS samples. Figure 14(b) represents the crack propagation. The reason for this type of crack propagation is due to the angular orientation at the time of fabrication. The fractograph is shown in figure 14(c) and a dimple region is observed which accounts for ductile behaviour [24, 25]. This type of ductile behaviour accounts for good mechanical properties [25]. Thus the Ti64 CS exhibits a ductile fracture behaviour which accounts for good compressive strength.
Figure 14. (a) CS Fabricated at 45° orientation (b) Crack propagation of the specimen (c) Fractograph of Ti64 CS.
Download figure:
Standard image High-resolution image3.4. Microstructural analysis
An optical microscope was used to analyse the microstructure of Ti64 CS. Initially, emery polishing of the sample was done. At first, grit papers of various sizes were used to polish the sample (according to the order of 180, 220, 320, 600, 800 and 1200). Next, fine emery papers were used to polish the sample using various grit sizes (according to the order of 1/0, 2/0, 3/0 and 4/0). Then, the sample was polished using a diamond cloth and cleaned using acetone. Finally, Keller's reagent was used to carry out preferential etching on the sample. The composition of Keller's reagent is 5 ml HNO3, 3 ml HCl, 2 ml HF and 190 ml H2O and it is an optimal etchant for titanium alloys [26]. The sample was finally washed in distilled water. Thus the sample preparation was done for microstructural analysis using optical microscope. Figure 15 shows the microstructure of the Ti64 CS.
Figure 15. Microstructure of Ti64 CS.
Download figure:
Standard image High-resolution imageThe microstructure reveals a very fine acicular i.e. plate-like morphology. The reason for this type of structure is due to the rapid cooling of titanium material after heat treatment i.e. there is a beta-to-martensite transition in the DMLS process during cooling. The heat treatment has a significant effect on microstructure and mechanical properties. There is a presence of both α phase and α'-martensite. However, because of the quicker solidification under cooling, the microstructure of the CS can be interpreted as martensitic [27, 28]. This type of microstructure exhibits high strength and low ductility. The microstructure of Ti64 has a significant impact on its mechanical properties [27]. Thus, the martensitic structure of CS is the reason for its good compressive strength.
3.5. Surface roughness analysis
In addition to microstructure, surface roughness is an essential parameter for biomedical applications. From the general perspective of tissue growth, a rough surface leads to better osseointegration. Simultaneously, too much rough surface may lead to bacterial adhesion on the implant [29]. Therefore it is desirable to have moderate surface roughness i.e. between 1 μm to 2 μm [30, 31]. Based on the requirement, the fabricated AM components are subjected to secondary post-processing in order to modify the surface finish according to the end medical application [32]. In this work, the quality of the CS surface was improved by means of the sandblasting technique. The non-contact surface roughness method was used to analyse the surface of the CS and the results are tabulated in table 7. The 3D surface of Ti64 CS is shown in figure 16. The average surface roughness Ra of the sample was measured to be 1.26 μm, which lies within the desirable range of surface roughness for medical implants [30, 31].
Table 7. Results of surface roughness analysis.
| Sample no. | Top | Bottom | Side 1 | Side 2 | Side 3 | Side 4 |
|---|---|---|---|---|---|---|
| Sample 1 | 3.62 | 1.97 | 0.916 | 0.862 | 0.663 | 0.592 |
| Sample 2 | 3.71 | 1.32 | 0.862 | 0.781 | 0.619 | 0.506 |
| Sample 3 | 2.58 | 1.04 | 0.890 | 0.713 | 0.607 | 0.480 |
Figure 16. 3D surface of Ti64 CS.
Download figure:
Standard image High-resolution image3.6. Biocompatibility study
The cell culture was performed, as discussed in section 2.7. Acridine Orange (AO) staining of MG-63 cells along with Ti64 CS was carried out and the morphology was analysed through the inverted fluorescent microscope. Following the staining with AO reagent, large green nuclei were observed in all live cells. This indicates that their cell membranes remained intact, thus confirming the cell viability. Figure 17(a) shows the clear intact nuclei, with green fluorescent proving the osseointegration property of Ti64 CS. Figure 17(b) denotes the percentage of cell viability during respective time periods i.e. day 1, day 3 and day 5. The results revealed that, there is an increase in the percentage of cell viability from 55% on day 1 to 68% on day 3 and 75% on day 5, respectively.
Figure 17. (a) Cell viability of Ti64 CS; (b) % of cell viability for three different time periods
Download figure:
Standard image High-resolution image4. Conclusions
In this research work, Ti64 CS was modelled as a honeycomb structure with variations in pore diameters and interpore distances. FEA results revealed that the CS model with 1 mm pore size and 1.6 mm interpore distance had the highest compressive strength and was fabricated through DMLS. The experimental results were in correlation with FEA results and they showed that the samples were capable of withstanding the required load for mandibular implant. Tissue culture was done in order to study the biocompatibility of CS. Following conclusions can be made from the work:
- (i)From FEA, the least von-mises stress was observed to be 48.67 MPa and the corresponding Young's modulus was calculated to be 34.76 GPa.
- (ii)Through compressive testing, the average Young's modulus was calculated to be 32.10 GPa, the average compressive strength was evaluated to be 51.25 MPa and the average strain energy was calculated to be 0.94 J, respectively.
- (iii)The compressive behaviour of Ti64 CS was observed to be sensitive to varying rate of loading.
- (iv)A ductile fracture was observed upon fractography analysis, which accounts for good compressive strength.
- (v)The CS exhibited a martensitic microstructure, which results in good compressive strength.
- (vi)The average surface roughness of the CS was measured to be 1.26 μm, which is within a suitable range of tissue growth for mandibular implant.
- (vii)The percentage of cell viability increases as 55% on day 1, 68% on day 3 and 75% on day 5, respectively.
Thus in this work, the mechanical characterization and biocompatibility study on additive manufactured Ti64 CS proves its suitability for application in mandibular implant. The implementation of CS reduces material consumption of the implant, thereby decreasing its weight compared to that of a solid implant.While still maintaining the same compressive strength, the cost of the implant shall be reduced.
Acknowledgments
The work described in this paper is supported by Grants (Senior Research Fellowship, File No. 09/468/0522/2018 EMR-I) from the Human Resource Development Group, Council of Scientific & Industrial Research, Ministry of Science and Technology, Government of India. The financial contribution is gratefully acknowledged.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).


















