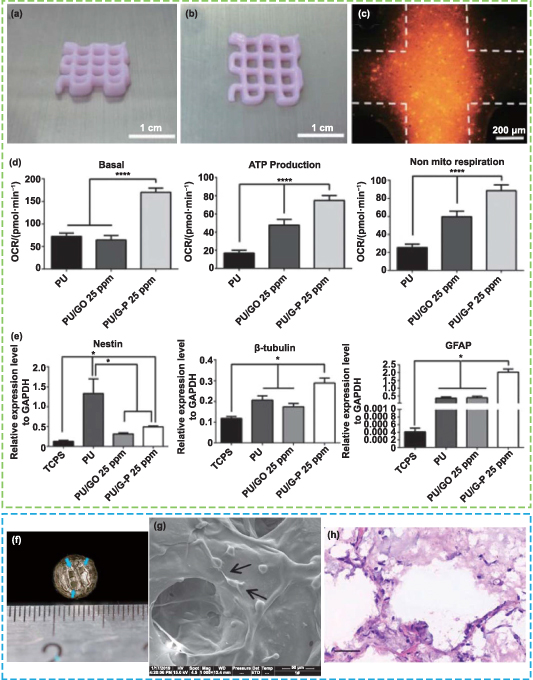
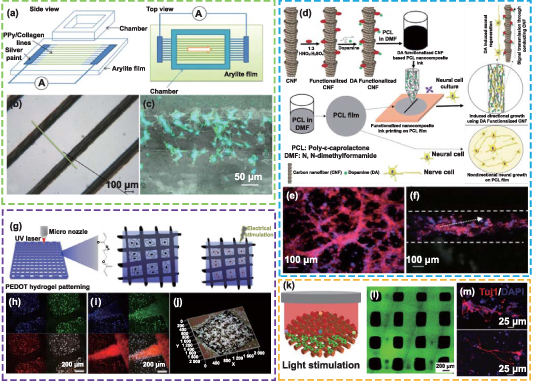
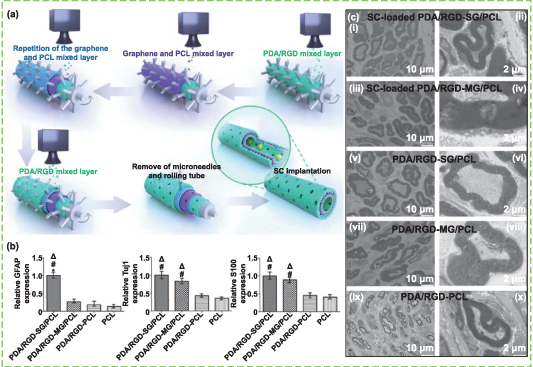
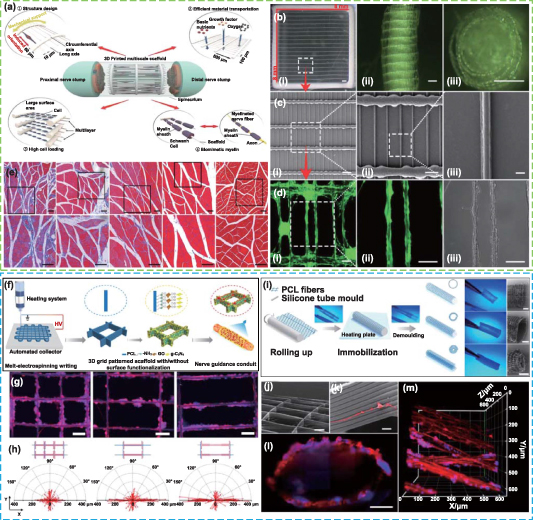
Abstract
Because of the complex nerve anatomy and limited regeneration ability of natural tissue, the current treatment effect for long-distance peripheral nerve regeneration and spinal cord injury (SCI) repair is not satisfactory. As an alternative method, tissue engineering is a promising method to regenerate peripheral nerve and spinal cord, and can provide structures and functions similar to natural tissues through scaffold materials and seed cells. Recently, the rapid development of 3D printing technology enables researchers to create novel 3D constructs with sophisticated structures and diverse functions to achieve high bionics of structures and functions. In this review, we first outlined the anatomy of peripheral nerve and spinal cord, as well as the current treatment strategies for the peripheral nerve injury and SCI in clinical. After that, the design considerations of peripheral nerve and spinal cord tissue engineering were discussed, and various 3D printing technologies applicable to neural tissue engineering were elaborated, including inkjet, extrusion-based, stereolithography, projection-based, and emerging printing technologies. Finally, we focused on the application of 3D printing technology in peripheral nerve regeneration and spinal cord repair, as well as the challenges and prospects in this research field.
Highlights
The anatomy of the nervous system, the current treatment strategies of the nerve injury, and the design elements of the nerve scaffold are outlined. It can be a tool for researchers to learn basic knowledge.
The latest progress of 3D printing technologies on the fabrication and function of nerve scaffolds are emphatically discussed, including inkjet, extension-based, stereolithography, and projection-based printing.
The advanced application of 3D-printed nerve constructs in peripheral nerve regeneration and spinal cord injury repair are emphasized, which can provide some new ideas for the tissue engineering nerve repair and regeneration.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The nervous system is the finest system of the human body with the most complex structure and function. It can be said that the nervous system is involved in the human body from head to foot, from inside to outside. The nervous system makes it possible for the body as a whole to coordinate and communicate to react to environmental changes [1]. The nervous system gathers information from cells in various body regions, analyzes the information, and then transmits signals to other cells and organs to cause the body to react as it should. The central nervous system (CNS) and the peripheral nervous system (PNS) are the two main divisions [2]. The CNS, which consists of the brain and spinal cord, is in charge of deciphering and processing information transmitted by nerves. The PNS is a type of broad neural network that spreads from the CNS to every part of the body. It transmits information between the CNS and the body [3]. Nerves in the PNS are channels that transmit sensory information to the CNS, and transmit information from the CNS to muscles and glands of the whole body [1]. The CNS and PNS jointly control sensory input, information integration, and movement output, forming the human nervous system [4].
The damage to the nervous system may be caused by ischemic, physical, chemical, mechanical, and other factors. Nerve system injury can lead to nerve transection and destruction of blood–nerve barriers, and finally cause pain, sensory disturbance, and physical and mental injuries [5, 6]. Nerve transection can lead to the interruption of communication between neurons and their supporting cells. All of these injuries may occur in the CNS and PNS, while, the responses of their neurons to the axotomy are fundamentally different [7]. The PNS has congenital regeneration potential after injuries, but for defects with a gap length greater than 10 mm, the nerve regeneration will not occur spontaneously, since fibrin cables and bands of Büngner fail to form under this condition [8]. Therefore, surgical intervention is necessary to resist the harsh microenvironment and bridge the nerve gap. At present, the clinical standard for the repair of long-gap peripheral nerve injury (PNI) is to use an autologous graft [8]. However, it has some limitations that must be considered, such as donor source shortage, size mismatch, and permanent damage to the donor site [9]. Unlike the PNS, the transected axons in the CNS can only produce abortive sprouting, which provides little functional recovery, and there is no effective clinical method to treat spinal cord injury (SCI) today [10, 11]. It is widely known that one of the main obstacles to the regeneration of the CNS is glial scar, which is mainly composed of reactive astrocytes and proteoglycans produced by them. Researches have demonstrated that inhibitory molecules, such as chondroitin sulfate proteoglycans (CSPGs) and myelin-associated inhibitors (MAIs), are the key factor to prevent the functional regeneration of neurons in the injured CNS [12, 13]. In addition to blocking the inhibitory molecules of neuron growth, enhancing the intrinsic regeneration ability of neurons to promote the functional recovery is one of the latest research trends in the treatment of axon regeneration in CNS [14–16]. As things stand, palliative functional recovery through pharmacological therapy and rehabilitation training was considered to be the best choice [17]. Unfortunately, these methods can neither reverse nerve injuries nor restore motor abilities.
Tissue engineering technology aims to replace, repair, or regenerate damaged, degraded, or defective tissues using engineering methods (including scaffolds, cells, and biophysical and biochemical cues) [18]. It has shown potential advantages in peripheral nerve and spinal cord regeneration [19]. Studies have shown that the tissue engineering structure could bridge the gap between the damaged nerves, and guide the regeneration of the broken axons [20]. The incorporation of cells can accelerate angiogenesis, promote myelin sheath regeneration and synaptic reorganization, and provide paracrine signal factors [21]. A variety of stimuli, such as chemical, mechanical, electrical, and topological cues, are also used to simulate the physiological microenvironment of the human body, facilitating nerve regeneration after PNI and SCI [22].
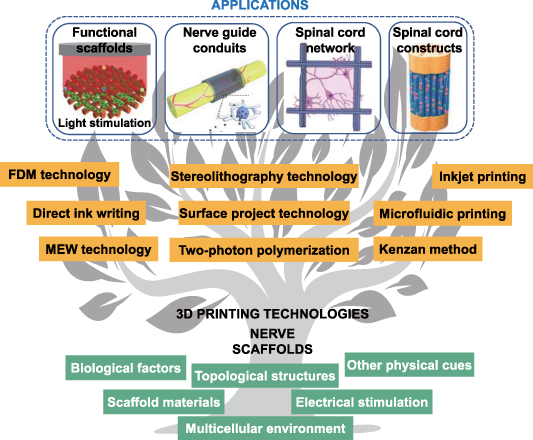
Among various elements, a scaffold should be the primary component of tissue engineering, since it provides a favorable environment for nerve cells and a three-dimensional (3D) platform for the regeneration of peripheral nerve and spinal cord [27]. An ideal neural scaffold should possess many physical, chemical, and biological properties, such as satisfactory biocompatibility, sufficient mechanical support, appropriate porosity and biodegradability, etc [20]. In this review, we emphasize the creation and function of nerve scaffolds with 3D printing (including 3D bioprinting). They show the potential advantages of structure bionics and function bionics in the regeneration of long-distance PNI and SCI. We first reviewed the anatomy of the natural peripheral nerve and spinal cord, the current treatment strategies for PNI and SCI, as well as the design elements of the nerve scaffold. Then, we emphatically discussed 3D printing technology that is applicable to neural tissue engineering and its latest progress, as well as its application in peripheral nerve regeneration and SCI repair, including inducing nerve differentiation, creating nerve guide conduits (NGCs), establishing spinal cord neural networks and constructing spinal cord-like scaffolds (figure 1). Finally, the challenges and prospects of 3D-printed nerve scaffolds in nerve repair and regeneration are emphasized.
Figure 1. Overview of the multiple design elements, 3D printing technologies, and typical applications involved in nerve scaffolds. Reproduced from [23]. © IOP Publishing Ltd. All rights reserved. Reprinted from [24], Copyright (2019), with permission from Elsevier. [25] John Wiley & Sons. [© 2021 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH]. Reprinted from [26], Copyright (2021), with permission from Elsevier.
Download figure:
Standard image High-resolution image2. Spinal cord and PNI treatment strategies
2.1. The spinal cord and peripheral nerve anatomy
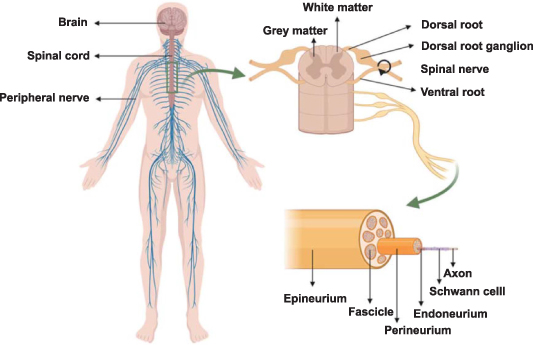
The brain and spinal cord are included in the CNS, which is one of two interacting subsystems that make up the human neurological system [28]. The vertebrae, intervertebral discs, muscles, and ligaments surround the spinal cord, which is housed in the spinal canal at the base of the spine [29]. It links the brain to different body components [30]. The spinal cord is a cylindrical organ with a length of 40–50 cm and a diameter of about 1–1.5 cm [28]. In general, its anatomy is dark butterfly gray matter in the middle and light white matter around (figure 2). The front two gray matter (anterior horn) contain neurons that transmit information from the brain to the PNS, whereas the latter two gray matter (posterior horn) do the reverse. The gray matter in the middle is the area where ganglia gather, and the white matter around is the area where nerve tracts gather. Nerve tracts in the white matter are parallel axonal bundles with similar functions, which are responsible for transmitting information from or to the brain [31]. In addition to neurons, the spinal cord contains neuroglia cells such as astrocytes, oligodendrocytes, and microglia. Among them, astrocytes have star-like morphology and the largest volume. Their main function is to maintain the stability of the chemical environment in the spinal cord [29]. Because astrocytes can produce and secrete some neurotransmitters and express some neurotransmitter receptors, they can react with some neuroactive substances to maintain the level of neurotransmitters. Meanwhile, astrocytes can biotransform exogenous compounds to help regulate the ionic microenvironment around neurons. In addition, there are many protrusions on the surface of astrocytes, which can stretch out and fill the cell body of each neuron and the gap between the protrusions, playing a supporting role. They can keep the structure of neurons stable and play a role of separation, so that the impulses between neuron cell bodies and their synapses will not be disordered. Oligodendrocytes can produce a kind of fat myelin to wrap axons, which makes electrical signal transmission more effective. Microglia cells are the resident immunoreactive cells in CNS and are responsible for clearing cell fragments like macrophages in PNS [29]. Similarly, blood vessels surround the whole spinal cord. There are 31 pairs of spinal nerves that originate from the spinal cord and are a part of the PNS [32]. There are dorsal and ventral roots for each pair of spinal nerves. The dorsal and ventral roots jointly form spinal nerves in the epidural space and penetrate the spinal canal from the space between the neural arches formed by the mesenchymal vertebrae. In terms of function, the dorsal roots are the afferent sensory roots, responsible for transmitting information to the brain, while the ventral roots are the efferent roots, responsible for transmitting information from the brain [33].
Figure 2. Schematic of the human spinal cord and peripheral nerve anatomy. Reprinted from [34] © 2021 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co. Ltd.
Download figure:
Standard image High-resolution imagePNS is made up of all nerves in the brain, spinal cord, and their related ganglia. The terms 'cranial nerves' and 'spinal nerves' refer to the nerves that emerge from the brain and spinal cord, respectively. PNS connects the spinal cord with various regions of the body, beginning with the spinal nerves, to sustain sensory and motor function. All peripheral nerves are made up of nerve fascicles [35], which are composed of supporting connective tissue and nerve fibers. Connective tissue surrounds nerve fibers longitudinally and has doubled function [36]. Firstly, it gives nerve fibers mechanical support and shields them from stretching and compression during movement of the body. Secondly, it possesses rich blood vessels (vasa nervorum) to ensure the nerve fibers with nutrient support and material exchange. Nerve fibers and connective tissue can be split into three layers from the innermost layer to the outermost layer, namely, the endoneurium, perineurium, and epineurium. Nerve fascicles are clumps of parallel axons that have been myelinated by Schwann cells (SCs). The endoneurium, a collagen matrix found inside the nerve, serves as protection for these fascicles (figure 2) [37]. The collagen matrix in this layer is loose. The perineurium is composed of collagen fibers and epithelium-like cell sheets [37]. It surrounds nerve fascicles and has strong mechanical strength. There are abundant longitudinally arranged vascular structures in this layer. Several nerve fascicles and vasa nervorum are further enclosed into the epineurium and isolated from the external environment. Vasa nervorum continues in this layer, and they are fully connected with the arteriole and venule network in the connective tissue deep in the nerve. Neurons are the basic cells in PNS. There are also several other important cell types in PNS, including SCs, satellite glial cells, macrophages, fibroblasts, and endothelial-like cells, which play an important role in axon regeneration [38–40]. SCs can produce extracellular matrix (ECM), cell adhesion molecules, neurotrophic factors, etc, providing a necessary living environment for the cells in PNS [39]. Satellite glial cells have similar functions to astrocytes in the CNS. They help regulate the chemical microenvironment in the PNS, and provide nutrients and structural support for the surrounding neurons [41]. Macrophages are willing to phagocytize the decomposed myelin sheaths and nerve fibers [38].
2.2. Spinal cord and PNIs
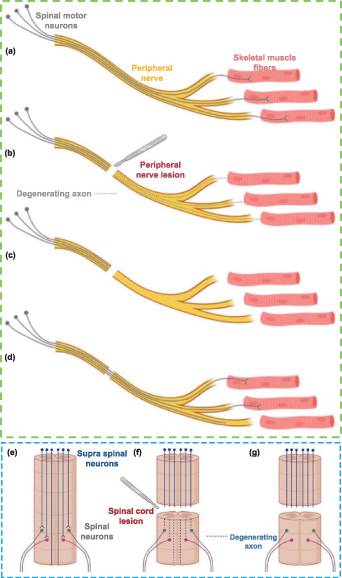
The loss of nerve structure and function caused by accidents, trauma, etc will lead to partial or total loss of sensory, motor, and autonomic capabilities as well as neuropathic pain, thus nerve injury is still one of the urgent clinical problems to be solved [42]. Nerve injuries can be roughly divided into non-degenerative and degenerative injuries [43]. The former has no axonal loss, while the latter has an axonal loss. To better understand the pathophysiology of nerve injuries, the researchers have proposed a more detailed classification of nerve injuries according to the presence of demyelination and the damage extent of the axons and the connective tissues, specifically including the neurapraxia, axonotmesis, and neurotmesis [44]. Neurapraxia is a mild injury caused by segmental demyelination, while axons and connective tissues are not damaged. Neurapraxia usually occurs when the nerves are slightly compressed or stretched. In this case, although the axons are complete, they have no function and cannot transmit impulses. In the case of axonotmesis, the axons are directly damaged or injured, and other anatomies in the peripheral nerve (such as endoneurium, perineurium, etc) are more or less damaged, but the surrounding connective tissues are complete. Neurotmesis is the most serious form of injury with nerve transection and loss of connective tissues, and the whole nerve trunk is damaged with the loss of continuity [43, 45]. In clinical practice, most nerve injuries are accompanied by various degrees of injuries, which is called a mixed injury pattern [46]. Figure 3 illustrated the axon degeneration or regeneration after nerve injury [47].
Figure 3. Schematic illustration of axon degeneration and regeneration after (a)–(d) peripheral nerve injury (PNI) and (e)–(g) spinal cord injury (SCI). Reproduced from [47]. CC BY 4.0.
Download figure:
Standard image High-resolution imageAfter SCI, a large number of cascade reactions are immediately triggered in the human body [48–50]. First, after the primary injury, neurons and neuroglia cells died and vasa nervorum broke in the injury site. Then the secondary injury goes on. During this period, inflammatory cells (including neutrophilic granulocyte, macrophages, microglia cells, and T cells) remove cell fragments and form glial scars to protect the injured spinal cord from further infection. The glial scar is helpful to the reconstruction of the blood supply system [51]. Once the damage is slowed down, self-healing will occur, including limited axonal sprouting and angiogenesis [52]. Although glial scars have advantages in preventing injuries, they hinder the regeneration of neurons, because of the existence of axon growth inhibitors (such as CSPGs and MAIs) in the ECM produced by scar-related cells [12, 51]. This is one of the reasons for the limited neuron regeneration after SCI.
The PNI can lead to partial or complete nerve transection, which is usually caused by trauma, infection, nerve-ending retraction, and compression ischemia. After PNI, an aseptic inflammatory reaction leads to fibrin deposition, fibrosis of the injury site and surroundings, scar formation, as well as the release of neurotoxic cytokines at the injury site. Axonal discontinuity causes injured neurons and other cells to phenotypically alter, which interferes with the creation of the myelin sheath and sets off the inflammatory cascade [53]. As a result, the second insult damages an increasing number of sensory and motor neurons (MNs). The distal end of the axon undergoes a series of pathologies, including swelling, myelin sheath destruction, and axon degeneration, which is also known as Wallerian degeneration [54, 55]. At the same time, macrophages are recruited to clean up myelin sheath and cell fragments. To repair the transected axons, cells express specific proteins such as neurotrophic factors and guide the regenerated axons to bridge the gap between the stumps, first forming a structure called a growth cone, and then gradually regenerating the axons from the proximal to the distal end. At the proximal end, axon sprouting and regeneration start from the bands of Büngner formed after the degenerative pathology, where SCs demyelinate and macrophages activate [56]. The bands of Büngner that are composed of longitudinally arranged SCs guide the regeneration of newborn axons to the distal end [57, 58], where functional re-innervation occurs in the target area [12]. The neurites can regenerate spontaneously along the bands of Büngner if the nerve continuity is not damaged. While, for severe or long-gap injuries, the significant inflammation and proximal disorder lead to the formation of dense scar tissue composed of SCs, macrophages, fibroblasts, and ECM, resulting in nerve adhesion and scar compression, and ultimately failure of axon regeneration [59, 60].
2.3. Current treatment strategies for SCI and PNI
The self-healing ability after SCI is very limited. During the stage of primary injury and inflammation, it was recommended to take pharmacological therapy, such as neuroprotective and anti-inflammatory agents (riluzole, minocycline, ketorolac, curcumin, etc) [61]. Among these medications, riluzole and minocycline are neuroprotective agents, which have been approved by the food and drug administration (FDA) for the treatment of some diseases, but have not been approved for the treatment of SCI. Nonetheless, several clinical trials utilizing riluzole or minocycline to treat SCI have been registered, and some published results have proved the feasibility and safety of these treatments, opening the door to clinical application [61, 62]. Ketorolac and curcumin possess neuroprotective effects due to their analgesic and anti-inflammatory properties, but their application in the treatment of SCI is currently limited to preclinical studies [63, 64]. In addition, decompression surgery can be conducted to remove the debris of vertebrae and intervertebral discs to stabilize the spine, tendon transfer can be applied to improve voluntary control of muscle and sensory function, and the blood pressure annotation method can also be used to protect neurons from further damage to prevent secondary damage [61]. In clinical trials, a large amount of effort has been devoted to studying the transplantation of stem cells (such as neural stem cells (NSCs), mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), etc) and neuroglia cells, expecting to improve the spinal cord regenerate during or after the secondary injury [65]. Nevertheless, the treatment effect of cell transplantations was discounted because of the low cell survival rate [61]. The researchers also proposed to combine cells and scaffold materials that can provide environmental protection and appropriate mechanical properties to enhance the survival rate of encapsulated cells. This method was expected to bridge the injured spinal cord and restore sensory and motor functions. Nowadays, some biomedical polymers have been used to fabricate scaffolds for the clinical trial of SCI treatment.
For mild PNI that does not damage nerve continuity, peripheral nerves can regenerate spontaneously after a sequence of pathophysiological reactions [20, 66]. However, the spontaneous regeneration process is insufficient to ensure proper functional recovery when the nerve continuity is damaged. Many factors affect the recovery of nerve function, such as the length of the nerve gap, the amount of time between the damage and treatment, and the patient's age [67]. In the 1960s, some feasible treatment methods began to be practiced in clinical, such as neurorrhaphy and other microsurgery [68]. Non-surgical and surgical treatments fall under two groups that make up PNI therapy options. The non-surgical treatments refer to the application of physical cues for rehabilitation therapy [69], such as electrical stimulation (ES) [70–72], magnetic stimulation [73], and laser phototherapy [74–76], which are helpful to eliminate inflammation and promote the absorption of edema at the early stage of injury, thus facilitating nerve regeneration. Although peripheral nerve regeneration has been demonstrated to be promoted by non-surgical treatments, further research is required to determine how well they work in long-gap (>3 cm) deficits. The best way of treatment for PNI is surgery [77], which includes neurorrhaphy, nerve grafting, and the application of tissue engineering scaffolds. The most common surgical treatment for short-gap (<1 cm) deficits is neurorrhaphy [78]. Generally speaking, the proximal and distal ends of the injured nerves were stitched together by surgical operation. While, for PNI with long-gap (>2 cm) defects, this method will lead to poor surgical effect due to the excessive suture tension [79]. The length of the transected nerve gap brought on by traumas has a significant impact on how well axons regenerate in living tissue. In most circumstances, spontaneous nerve regeneration will not take place when the crucial gap length is exceeded. Nerve grafting and artificial nerve guide conduits (NGCs) implanted between transected segments are all possible treatments for PNI. The most popular method for treating middle- and long-gap PNI is nerve grafting. There are three important terms related to nerve grafting, that are allografts, nerve transfers, and autografts. Allografts have the advantages of unlimited donors, avoiding donor site morbidity, and requiring temporary immunosuppression, thus they become one of the major therapies for PNI repair [80]. The main limiting factors of allografts are the side effects of immunosuppression, such as metabolic disorders, malignant tumors, opportunistic infections, etc [81]. The best treatment for brachial plexus injuries that cause significant upper limb dysfunction and disability is nerve transfer [82], however, this treatment is not yet known for PNI in other areas of the body. In addition, this therapy has some limitations, such as the pre-existing injury and morbidity of donor nerve as well as co-contraction [83]. Autograft has been considered the gold standard for the repair of long-gap PNI [78, 81]. Compared with all other therapies, autograft provides the best nerve regeneration effect up to now [84]. However, in autologous transplantation, functional sensory nerves were usually used as donors to repair the damaged motor nerves, which would lead to the morbidity of donor sites, including sensory loss, scar tissue, and neuroma formation [85]. Moreover, autograft also has some other disadvantages, such as limited donors, secondary surgical trauma, inevitable tissue size mismatch, and loss of function [78, 86]. To break the limitations of autologous nerve grafts, artificial NGCs constructed by tissue engineering method has received increasing attention, which could be the most promising substitute. The FDA has currently approved a number of synthetic scaffolds comprised of collagen (NeuraGen®, NeuroMatrix®, etc.), polyvinyl alcohol (PVA) (SaluChannel®), polyglycolic acid (PGA) (NeuroTube®), and other materials [67], but their application is still restricted to nerve deficits shorter than 3 cm.
3. Design elements of scaffolds for peripheral nerve and spinal cord
Nerve regeneration is a complex physiological process, in which the interactions of cell–cell and cell–ECM play an important role. Using tissue engineering methods, natural and/or synthetic biopolymers are prepared and designed into tubular structures with mechanical and biochemical properties necessary for nerve regeneration, which are called NGCs. NGCs can overcome the limitations of neurorrhaphy and nerve grafting [87]. As a bridge between damaged nerve stumps, the NGC provides the required structure and nutrition support for both ends, assists the surrounding tissues to infiltrate, and guides axon regeneration along the conduit. NGCs generally refer to artificial nerve conduits and artificial nerve grafts. An ideal NGC should possess a bionic structure, topological characteristics that can longitudinally arrange regenerated axons, sufficient mechanical properties to provide structure support, adequate permeability to provide nutrition support, electrical conductivity, flexibility, as well as appropriate biodegradability to allow free elongation, diffusion, and signal transmission of cells [78, 88]. In 1982, the first NGC was fabricated as a non-bioabsorbable silicon tube and applied to repair a 6 mm nerve gap [89]. Although the ideal NGCs have not been obtained yet, this will not prevent more and more researchers from proceeding with more comprehensive development.
So far, researchers have proposed a variety of strategies in the synthesis and modification of biopolymers, the integration of cells and bioactive molecules, the application of physical stimulation, and the gene manipulation of target cells. These strategies can be combined to develop ideal NGCs. It is worth noting that technological progress has brought about noticeable changes in the development, preparation, and selection of biopolymers for NGCs. Similarly, many studies showed that the biological performance of NGC loaded with cells has improved compared with that of empty NGC. Early research mainly used mature cells, while advances have been made in exploring varieties of stem cells for nerve regeneration today. Nevertheless, the types, sources, and integration methods of cells in NGC are still an unresolved problem. In the past decades, researchers have carried out extensive exploration to address these challenges.
To simulate the conditions in vivo, different neurotrophic factors were compounded into NGCs. The results showed that up-regulating the expression of some neurotrophic factors can promote the regeneration of injured nerves. Significant advancements have been made in neurotrophic factor loading and release methods during the past few decades. A successful nerve graft must also have the capacity to direct the sprouted axons toward the distal end of the transected nerve and to provide enough cues to aid in their re-entry into the appropriate fascicle, promoting the re-innervation of the target organs [90]. Therefore, it has been reported that axon growth can be guided by providing physical/external stimuli. These cues have been demonstrated to be useful in directing the regenerated axons to travel to the target fascicle and then the desired innervation sites. For effective nerve regeneration to be stimulated and finally accomplished, these many techniques must be integrated.
3.1. Scaffold materials
For tissue regeneration, scaffold materials play a crucial role in the process of constructing a microenvironment that allows cells and tissues to grow [91]. Biocompatibility, biodegradability, and the requisite mechanical qualities are the key characteristics that apply to scaffold materials used in tissue engineering [92, 93]. Natural and synthetic polymers are the materials that are utilized the most frequently. Alginate, cellulose, hyaluronic acid (HA), chitin, and chitosan are examples of polysaccharide-based natural polymers, while collagen, fibrin, fibronectin, laminin, Matrigel (MA), and gelatin are examples of protein-based natural polymers [94, 95]. Most synthetic and natural polymers have been applied to fabricate NGCs [96]. Keilhoff et al implanted type I/III collagen conduits loaded with SCs in the 20 mm sciatic nerve space of rats, and found the conduits were vascularized and integrated with the host tissue within 5–7 d after implantation [97]. Bozkurt et al prepared highly oriented 3D collagen scaffolds by freeze-drying technology, and the results in vitro showed that this collagen scaffold can guide axon regeneration [98]. In another study, Suzuki et al used freeze-dried alginate gel to bridge the 7 mm sciatic nerve gap in rats [99], and the results in vivo proved that the sensory and motor nerves in the injured tissues were functionally reinnervated. Although natural polymer materials have made some achievements in nerve injury repair, their mechanical properties are poor and it is difficult to construct 3D structures. Therefore, researchers mainly used synthetic polymers to fabricate NGCs, such as poly( -caprolactone) (PCL), polyurethane (PU), polylactic acid (PLA), PGA, poly(lactide-co-glycolide) (PLG), and poly(3-hydroxybutyrate) (PHB). Reid et al implanted the PCL nerve conduit into the 10 mm sciatic nerve gap in rats [100]. At 18 weeks after implantation, compared with the autologous nerve graft, the PCL conduit promoted the axon regeneration of similar volume and the same number of myelinated axons at the distal stump. However, the degradation rate of PCL is relatively slow, and may last for 2–4 years, which is not satisfactory [101]. PLGA is a combination of PLA and PGA, and its degradation rate can be controlled by adjusting the proportion of PLA and PGA [102]. The higher the content of PGA, the faster the degradation rate. Some studies had shown that a thin tissue capsule, and fibrin matrix cable were respectively formed on the outer and inner surface of the micro-braided PLGA conduits which were implanted into the 12 mm sciatic nerve gap of rats, indicating good biocompatibility of the NGCs [103]. Although lactic acid and glycolic acid, which are the by-products of PLGA degradation, are non-toxic, they are relatively acidic and difficult to metabolize in large quantities [104]. PU has good flexibility and by adjusting the mechanical properties and degradation rate of PU-based polymers, Hsu et al synthesized waterborne biodegradable PU for fabricating nerve conduits [105]. As comparison to commercial Neurotube, the PU conduit demonstrated superior nerve regeneration after implanted into the 10 mm sciatic nerve gap in rats for 6 months.
-caprolactone) (PCL), polyurethane (PU), polylactic acid (PLA), PGA, poly(lactide-co-glycolide) (PLG), and poly(3-hydroxybutyrate) (PHB). Reid et al implanted the PCL nerve conduit into the 10 mm sciatic nerve gap in rats [100]. At 18 weeks after implantation, compared with the autologous nerve graft, the PCL conduit promoted the axon regeneration of similar volume and the same number of myelinated axons at the distal stump. However, the degradation rate of PCL is relatively slow, and may last for 2–4 years, which is not satisfactory [101]. PLGA is a combination of PLA and PGA, and its degradation rate can be controlled by adjusting the proportion of PLA and PGA [102]. The higher the content of PGA, the faster the degradation rate. Some studies had shown that a thin tissue capsule, and fibrin matrix cable were respectively formed on the outer and inner surface of the micro-braided PLGA conduits which were implanted into the 12 mm sciatic nerve gap of rats, indicating good biocompatibility of the NGCs [103]. Although lactic acid and glycolic acid, which are the by-products of PLGA degradation, are non-toxic, they are relatively acidic and difficult to metabolize in large quantities [104]. PU has good flexibility and by adjusting the mechanical properties and degradation rate of PU-based polymers, Hsu et al synthesized waterborne biodegradable PU for fabricating nerve conduits [105]. As comparison to commercial Neurotube, the PU conduit demonstrated superior nerve regeneration after implanted into the 10 mm sciatic nerve gap in rats for 6 months.
Synthetic polymers have good mechanical properties, but poor cell affinity. In order to make full use of the advantages of distinct polymers, synthetic and natural polymers or a variety of synthetic polymers were compounded to prepare tissue engineering scaffolds, which integrated the excellent and unique characteristics of the component polymers and possessed more suitable and comprehensive performance for nerve regeneration. Yu et al compounded PCL with collagen and prepared NGCs by electrospinning [106]. In vitro cell study revealed that collagen/PCL composite fibers promoted the adhesion and proliferation of SCs. When the collagen/PCL conduits were implanted into the 8 mm sciatic nerve gap of rats, they showed similar electrophysiological characteristics and re-innervation results compared with the autograft. At 4 months after implantation, although the regenerated nerve fibers were still immature, the collagen/PCL conduit promoted more axon regeneration and gradually degraded, which was matched with the nerve regeneration rate. Quigley et al reported a kind of multicomponent and multimodal synthetic conduit composed of PLA/PLGA/alginate hydrogel, and used it to promote SCs migration and axon regeneration [107]. After 4 weeks of sciatic nerve implantation in rats, compared with the group of the conduit containing only alginate, the rats in the full-configuration conduit group represented significantly less over-grooming and autotomy of the limb, indicating that the full-configuration conduit could transmit some feelings back to the limb. Immunohistochemical analysis showed that myelinated axons appeared in both the conduit and the distal end of the implantation site, further proving the full-configuration conduit promoted nerve repair.
3.2. Topological structure
The peripheral nerve and spinal cord contain many fascicles composed of parallel axons, which are respectively surrounded by SCs and oligodendrocytes to form myelin sheaths. To effectively restore tissue function, the nerve tissue engineering scaffold must provide a topology similar to natural ECM, endowing cell direction and guiding axon growth and reconnection [108]. It was reported that the scaffold with longitudinal conformation could provide contact guidance and space constraint to regulate and control the growth and arrangement direction of neurons [109]. In addition, growing axons prefer ordered micro/nano features rather than randomly oriented topology, and the former can promote the growth of axons from the proximal to the distal end [110]. The researchers designed NGCs with aligned pores/microchannels by unidirectional freeze drying, arrays of ordered microtubes or linear micropatterns with ridges and grooves, achieving remarkable results in guiding nerve cell migration and neurite extension [111–114]. In order to mimic the channels around or inside natural nerves, researchers designed NGCs with macro- or micro-scopic lumens and implanted them into the injured nerve gaps. Compared with the conduits with hollow lumens, the conduits with micro/nano features (such as nano roughness, nano grooves, micropore/microgroove, silica beads, micropillar arrays, etc) in the lumens showed excellent functional nerve regeneration ability [115–119].
Besides, considering the diffusion and mass transfer of nutrients, metabolites, gases, wound exudates, etc in the scaffolds, researchers designed NGCs with different types of pore structures on the outer wall, including porous, nonporous, and semi-porous NGCs [120]. In vitro and in vivo studies showed that NGCs with semipermeable/asymmetric porous outer walls were considered the best among the three types of conduits, because they not only allowed diffusion mass transfer and blood vessel invasion, but also prevented fibroblast infiltration.
3.3. Multicellular environment
For the regeneration of the spinal cord and peripheral nerves, there are primarily two different types of tissue engineering scaffolds. One is a scaffold that has cells incorporated into it, which involves first seeding or embedding cells on or into the scaffold in vitro, and then grafting the scaffold-cell complex into the location of the damage [21, 121]. The incorporated cells secrete multiple growth factors, signal molecules, and ECM molecules, which are necessary for axon regeneration. The other is the scaffold without cells but with growth factors or other biological molecules, which can be released and in situ recruit autologous cells [109].
In nerve regeneration, commonly used supporting cells include mature cells, stem cells, and genetically modified cells. In PNS, SCs are the main glial cells, which wrap around axons to form the myelin sheath, and secrete neurotrophic factors that can promote nerve regeneration [122]. After PNI, SCs migrate, arrange and self-organize into bands of Büngner in the damaged area. Berrocal et al encapsulated SCs into collagen conduits to bridge the 13 mm sciatic nerve gap of rats. It was found that collagen conduits loaded with SCs and autografts facilitated the regeneration of similar numbers of myelinated axons after 16 weeks of surgery [123]. However, compared with the autograft group, the autotomy of the limb of rats in the SCs conduit group was significantly reduced, proving that the conduits filled with SCs significantly increased the repairable distance after long-gap PNI. Some studies have also shown that incorporating SCs is conducive to repairing the injured spinal cord [124, 125]. After implantation for a long time (3, 5–7, and over 8 weeks), it can significantly improve the motor function recovery of rats with traumatic SCI. Neuroglia cells in CNS, such as astrocytes and oligodendrocytes, have been used in some preclinical studies of spinal cord regeneration, and have greatly improved functional recovery after SCI [126–128].
Stem cells, such as NSCs, MSCs, HSCs, pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) or induced PSCs (iPSCs), will eventually differentiate into neuron-like cells with the effect of nerve growth factor (NGF) [129]. Jia et al used an acellular allograft loaded with bone MSCs (BMSCs) to bridge the 10 mm sciatic nerve gap of rats [130]. After 8 weeks of surgery, the nerve regeneration and function recovery in the group of the allograft loaded with BMSCs were better than those in the empty acellular allograft group, but was still inferior to the autograft group. Zhang et al loaded neural differentiated adipose-derived stem cells (ADSCs) into xenogeneic acellular nerve matrix (XANM), and grafted them into the 10 mm sciatic nerve gap of rats [131]. Before implantation, ADSCs were cultured in XANM for 4 d in vitro, inducing differentiated ADSCs (dADSCs) to produce a longitudinal cell alignment along the XANM axis and express genes related to neurotrophic factors (NGF, glial cell line-derived neurotrophic factor (GDNF), and brain-derived neurotrophic factor (BDNF)). In vivo studies showed that the grafts loaded with dADSCs and SCs had comparable effects on nerve regeneration. Some studies have also shown that transplantation of iPSCs was conducive to repair SCI [132], and significantly improved the motor recovery of rats with SCI [133]. Yang et al found that after subacute spinal cord compression injury, repeated intravenous injection of human umbilical cord blood-derived MSCs (hUCBMSCs) in two discontinuous segments could facilitate the recovery of nerve function. During this process, hUCBMSCs differentiated into specific cell types, with reduced inflammation, apoptosis, and astrocytoma, as well as enhanced axon protection [134]. Fan et al encapsulated iPSC-derived NSCs (iNSCs) into 3D gelatin methylate (GelMA) hydrogel and implanted it into a mouse spinal cord transection model for nerve regeneration [135]. It was found that GelMA/iNSC grafts inhibited the growth of glial fibrillary acidic protein (GFAP) positive cells and the formation of the glial scar, and ultimately promoted axon regeneration.
It is well known that gene manipulation can promote the long-term production of growth factors at target sites [136]. Some studies have shown that the transfected NSCs have excellent proliferation and differentiation abilities, and can maintain the sustained release of NGF during the culture process. Shi et al transfected NSCs with lentivirus vector by GDNF gene [137]. Compared with the NSC incorporated or pure conduit groups, the PLGA conduit loaded with transfected NSCs significantly promoted the growth of facial nerve in rats, mainly in terms of nerve conduction velocity, as well as the area and the number of the regenerated axon. Considering the mutation risk of lenti- and retro-viral vectors, it is also an advisable choice to transfect SCs by liposome-mediated GDNF. Zhou et al loaded the SCs transfected with the GDNF gene into the PLGA conduit and used it to bridge the 5 mm facial nerve gap of rats [138]. Compared with the suspended SC and direct anastomosis groups, the transfected SC group showed better nerve regeneration effects.
3.4. Addition of neurotrophic factor
In addition, the introduction of neurotrophic factors and other growth factors involved in nerve regeneration can also effectively facilitate cell behavior and function [109]. NGF, glial growth factor (GGF), ciliary neurotrophic factor (CNTF), BDNF, GDNF, and neurotrophin-3/4/6 (NT-3/4/6) are commonly applied [86, 139, 140]. Through particular transmembrane receptors like tropomyosin receptor kinase and p75 [141], neurotrophic factors act to activate the cascade reaction of downstream signal pathways, thereby facilitating the functions of the neuron [142]. To maintain the activity of neurotrophic factors, they are usually loaded into the scaffolds by physical or chemical means, such as directly suspending into the lumen of NGCs [143], entrapping into the outer wall of NGCs [144], encapsulating into microspheres [145], entrapping into hydrogels and fibers [146], cross-linking with gels [147], binding with heparin [148], and loading in the form of recombinant proteins [149]. Compared with directly suspending or entrapping neurotrophic factors into NGCs, encapsulating them into microspheres and then compounding them into NGCs can achieve sustained release of neurotrophic factors. In order to evaluate the long-term release effect of neurotrophic factors, Kokai et al compounded GDNF into PLGA/PLA double-walled microspheres, and then entrapped them into the outer wall of PCL nerve conduits [145]. The results showed that the double-wall microspheres inhibited the typical explosive release, as well as the release rate was 2.9% within 3 d, 89.0% within 56 d, and the rest was completely released within 112 d. Compared with the control group, the nerve count, gastrocnemius contractility and tissue integration of the GDNF-treated group were better after implanting the NGCs into the 15 mm sciatic nerve space of rats for 16 weeks. Bi-domain heparin-binding peptides, heparin, NGF, or GDNF were loaded into the fibrin matrix by Wood et al, who also proposed an affinity-based delivery system (ADS) [150]. Heparin creates links with bi-domain peptides and neurotrophic factors (NGF or GDNF) in this ADS, whereas bi-domain heparin-binding proteins make bonds with the fibrin matrix and heparin [151]. The researchers implanted the silicon tube loaded with ADS fibrin into the 13 mm sciatic nerve gap of rats. At 6 weeks post-surgery, it was found that the conduits containing heparin-binding GDNF or NGF displayed similar effects on nerve fiber density, nerve tissue percentage, and myelin sheath area with the allografts.
3.5. ES
The axons transmit information through electrical pulses under the physiological conditions of PNS and CNS. The cell membrane possesses bioelectricity, which can form weak electric fields locally, and these electric fields will be strengthened by conductive scaffolds under the mutual stimulation of adjacent cells, finally affecting cell proliferation and differentiation [152]. ES has been shown in numerous studies to encourage nerve regeneration and functional recovery after damage [153–156]. Researchers have explored a variety of conductive polymers and piezoelectric polymers for fabricating conductive scaffolds, such as polypyrrole (PPy), polyaniline (PANI), polythiophene (PTh) and its derivatives poly (3,4-ethylenedioxythiophene) (PEDOT) [157], poly (vinylidene fluoride) (PVDF) [158], carbon nanotubes [159], MXenes [160, 161], as well as graphene and its derivatives [162–165]. The composite of PPy into the scaffold not only increases the proliferation of rat phaeochromocytoma cells (PC12 cells), but also accelerates the formation of axonal myelin sheath [166]. Song et al prepared PPy/poly (l-lactic acid-co- -caprolactone) conductive nanofibers by electrospinning technology, and constructed NGCs that were implanted into 15 mm sciatic nerve gap of rats [167]. The results showed that with the ES, the effect of nerve regeneration in the conductive polymer group was similar to that in autograft group. Abidian et al coated PEDOT on an agarose gel to prepare hydrogel-based NGCs, and implanted them into the 10 mm peroneal nerve gap of rats [168]. After 12 weeks of surgery, the morphology of the regenerated nerve in the hydrogel-based NGC groups was similar to that in the autograft groups. To evaluate the role of carbon nanotubes in axon regeneration, Yu et al constructed multi-walled carbon nanotube (MWCNT)-enhanced collagen/PCL nerve conduits, and implanted them into the 8 mm sciatic nerve gap of rats to repair the defect [169]. The results showed that conductive nerve conduits can perfectly regenerate nerves between gaps without causing immune rejection or severe inflammation.
-caprolactone) conductive nanofibers by electrospinning technology, and constructed NGCs that were implanted into 15 mm sciatic nerve gap of rats [167]. The results showed that with the ES, the effect of nerve regeneration in the conductive polymer group was similar to that in autograft group. Abidian et al coated PEDOT on an agarose gel to prepare hydrogel-based NGCs, and implanted them into the 10 mm peroneal nerve gap of rats [168]. After 12 weeks of surgery, the morphology of the regenerated nerve in the hydrogel-based NGC groups was similar to that in the autograft groups. To evaluate the role of carbon nanotubes in axon regeneration, Yu et al constructed multi-walled carbon nanotube (MWCNT)-enhanced collagen/PCL nerve conduits, and implanted them into the 8 mm sciatic nerve gap of rats to repair the defect [169]. The results showed that conductive nerve conduits can perfectly regenerate nerves between gaps without causing immune rejection or severe inflammation.
The current conductive or piezoelectric polymers used are not biodegradable, despite the fact that conductive scaffolds are advantageous for nerve regeneration. As a result, their use in nerve regeneration is restricted [170], and their significance in clinical transformation also needs to be carefully considered.
3.6. Mechanical stimulation and other physical cues
In addition, the stiffness and viscoelasticity of scaffold materials will also be considered to match the characteristics of nature tissues [171]. For example, the harder network will inhibit the proliferation and elongation of SCs, as well as the extension of neurites [172, 173]. The potential mechanism was supposed to be the mechanotransduction mismatch between matrix and cells, which ultimately changed cell phenotype, proliferation, and differentiation [174]. Fan et al prepared three hydrogels of variable stiffness by controlling the UV exposure time. They found that iNSCs embedded in low-modulus hydrogels could survive and differentiate better. Moreover, the softer hydrogel showed enhanced neurite growth and more neuron differentiation [135]. Mei et al fabricated a series of soft and elastic 3D hydrogels, and discussed the influence of mechanical stretching on the behavior of nerve cells [175]. It was found that mechanical stretching can significantly promote neurite extension and axon elongation, and the axons are oriented along the stretching direction.
In recent years, other external forces, such as ultrasound and magnetic stimulation [176], have also been applied in nerve repair. As a new non-invasive physical stimulation, external ultrasound is proposed because it can stimulate SCs cells and activate neurotrophic factors. It is reported that low-intensity ultrasound stimulation significantly increased the speed of nerve regeneration and promoted the regeneration effect. Park et al implanted the PLGA/Pluronic F127 nerve conduits into the 10 mm sciatic nerve gap of rats, and used low-intensity pulsed ultrasound to stimulate percutaneously the implanted site [177]. One week and two weeks after surgery, immunohistochemistry analysis showed that compared with the nerve conduits without ultrasound treatment (0.48 mm d−1), the nerve conduits with ultrasound treatment facilitated the regenerative axons to reach the distal stump more quickly (0.71 mm d−1). In addition, 4 and 8 weeks after surgery, the histological evaluation showed that the ultrasonic (US) stimulated nerve conduit groups had a better repair effect than the unstimulated conduit groups, mainly reflected in the more extensive area of regenerated nerve tissue, larger axon diameter, and thicker myelin sheaths.
4. 3D printing technologies applied in nerve tissue engineering
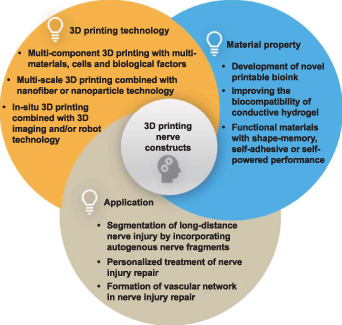
Although traditional technologies have shown some success in fabricating nerve tissue scaffolds, they are still limited in simulating the organization of structures and cells in vivo. 3D printing provides the requisite spatial accuracy to design the scaffold, which can simulate the structure and material flexibility to match the mechanical and chemical properties of natural tissues. The term '3D printing' refers to a group of adaptable additive manufacturing (AM) techniques that can precisely build constructs with intricate 3D characteristics. It offers excellent benefits in terms of design adaptability, personalization, structural dependability, and a variety of usable materials [178]. A 3D construct is built layer by layer using curable materials and a computer-controlled working system, all while being guided by specified digital models. It can easily obtain elaborate geometric distribution containing biomaterials, multiple cells, and/or growth factors, as well as can provide personalized patient-specific treatment options. This section mainly outlines the widely used 3D printing methods in nerve tissue engineering, including inkjet printing, extrusion-based 3D printing, stereolithography (SLA), surface project technology, and several emerging 3D printing methods. Their respective advantages and disadvantages are shown in table 1.
Table 1. Advantages and disadvantages of different 3D printing technologies in fabricating scaffolds.
| Technology | Advantages | Disadvantages |
|---|---|---|
| Inkjet printing |
|
|
| FDM technology |
|
|
| Direct ink writing |
|
|
| MEW technology |
|
|
| SLA technology |
|
|
| Projection-based printing |
|
|
| TPP technology |
|
|
| Microfluidic printing |
|
|
| Kenzan method |
|
|
Abbreviations: FDM, fused position melting; MEW, melt electrowriting; SLA, stereolithography; TPP, two-photon polymerization.
4.1. Inkjet printing
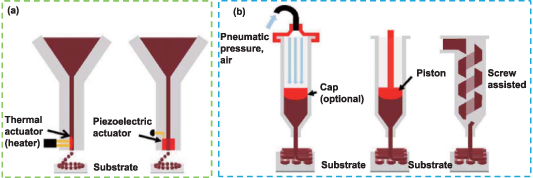
Using layer-by-layer (LBL) accumulation, inkjet printing is a 3D manufacturing process that can deposit minute solution droplets along the x, y, and z axes in a highly controllable manner to produce the desired pattern on the substrate. The ink ingredient must be kept liquid throughout the printing process to form droplets, harden immediately after deposition, and finally result in a 3D structure [179]. Compared with other printing methods, inkjet printing possesses a smaller deposition volume, faster printing speed [180], and higher printing resolution (∼50 µm) [181]. Inkjet printing is a very attractive and relatively inexpensive technology, which combines data-drive and non-contact approaches, and enables precise volumes of ink to be deposited at the target location in a high-speed and accurate manner. There are many types of inkjet printing, which can be based on thermal and piezoelectric mechanisms (figure 4(a)) [182]. In thermal inkjet printing, the electrically heated jet head generates an air pressure pulse to drip the ink from the nozzle. Radulescu et al discussed the 'jettable' parameters of PLA/PCL inks that were dissolved in different solvents (such as chlorinated organic compounds and cellular acetate) with concentrations ranging from 1% to 10% (w/v) and ultimately fabricated cylindrical nerve conduits, which has the ability to maintain cell adhesion, growth and secretion of NGF [183].
Figure 4. Schematic representation of (a) inkjet and (b) extrusion-based printing. Reprinted from [182], Copyright (2020), with permission from Elsevier.
Download figure:
Standard image High-resolution imageLiquid bio-ink, which can simultaneously deposit various materials and cells and precisely place them at the micron-scale resolution, has been handled using inkjet technology for bioprinting with success. There are several benefits to inkjet bioprinting in terms of print quality. Drop-on-demand inkjet printing has been shown to precisely deposit cells while maintaining their vitality [184]. Additionally, inkjet printing can precisely arrange cells to encourage the development of delicate neural networks [185]. Xu et al created a complex and heterogenous 3D construct by printing layer by layer three different types of cells in the pre-determined locations of the sodium alginate/collagen matrix [186]. The resulting 3D constructs embedded cells can enhance cell proliferation, differentiation, and maturation into functional tissues in vivo.
The main problem of inkjet printing technology is that cells or materials may block the nozzle of the print head, which may cause thermal stress, mechanical stress, etc, thus damaging cells. The ink must have a low viscosity and low cell density to prevent nozzle obstruction. Other limitations in the printing process result from the regulation of these parameters (such as droplet diffusion, cell sedimentation, etc). Although most cells maintained healthy and normal proliferation, several recent research indicated that there was no discernible difference in cell viability between printed and non-printed cells [186, 187]. Tse et al printed SCs and neuronal analogue NG108-15 cells using piezoelectric inkjet technology [187]. After printing, the viabilities of glial cells and neuronal cells were respectively higher than 90% and 86%, which had no correlation with the applied voltage. Compared with the non-printed cells, the printed neural cells produced longer synapses earlier. This method of inkjet printing cells can be used to investigate the interactions of neuron–glial cells, and there is no significant difference with the standard cell seeding in cell viability. These results provided a broad platform for nerve regeneration methods.
4.2. Extrusion-based printing
Extrusion-based printing is realized by depositing materials layer by layer through a nozzle on the movable print head under the control of a computer (figure 4(b)). It can be divided into melt- and solution-based processes. The majority of viscous molten or semi-molten polymer slurries, solutions, or dispersions are used for printing [188]. One of the most often used methods for producing 3D structures with encapsulated cells is extrusion-based printing, which employs a piston or screw to constantly extrude bio-ink. The primary causes of cell damage are pressure and shear force produced by extrusion, hence it is important to optimize the printing parameters (bio-ink viscosity, cell density, ambient temperature, air pressure, etc) to avoid cell inactivation [185]. The primary benefit of this bioprinting technique is that it can print viscous bio-ink with high cell density, which is difficult for conventional printing technologies to accomplish. Extrusion-based bioprinting, on the other hand, has a lower ability to correctly reprogram the printing shape and cell sites when compared to other printing techniques. It also has a slightly worse geometrical resolution. A novel aqueous PU hydrogel with thermoresponsive properties was prepared by Ho and Hsu [189]. They used extrusion-based bioprinting to load Forkhead box D3 (FoxD3) plasmids and human fibroblasts together onto the 3D-printed PU hydrogel scaffold. The results showed that fibroblasts were reprogrammed in situ after induction and differentiated into neural tissue-like constructs, which could be applied in nerve regeneration. The commonly used extrusion-based printing processes mainly include fused position melting (FDM), direct ink writing and melt electrowriting (MEW).
4.2.1. FDM technology.
One of the popular 3D printing technologies is FDM. The thermoplastic filament materials are extruded using a temperature-controlled extruder, and the semi-molten polymers are then layer by layer deposited on the printing platform. This approach is distinctive in that it offers excellent convenience and flexibility in material handling and processing while not requiring any solvent. A scaffold with a very uniform internal honeycomb structure, customizable pore morphology, and full connectivity between pores can be created by altering the material deposition method of the continuous deposition layer and the deposition spacing of any layer. By using filament-like materials, production may continue without having to switch materials because the residence time in the heating chamber is reduced [190]. There are many kinds of materials suitable for FDM, but the printing resolution is limited, about 100 µm [180]. Hsiao et al extruded molten PLA filament from a nozzle with a diameter of 0.25 mm at 195 °C to obtain 3D scaffolds with different gap widths between struts [191]. FDM realized the diversity of scaffold structure, and its micro-scale porous isotropic fibers induced human dental pulp stem cells to show morphological change and neural differentiation. Uz et al fabricated 3D gelatin scaffolds with graphene-based interdigitated circuits, which had tailored 3D microarchitecture and mechanical properties [192]. Under the effect of external ES, 80% of MSCs cultured on 3D nerve scaffolds expressed SC markers, and the secretion of NGF was significantly increased, indicating that ES applied within the 3D conductive scaffold could enhance the neural differentiation and paracrine activity of MSCs. These studies highlight the feasibility of using FDM technology to construct nerve tissue engineering scaffolds.
The disadvantage of FDM technology is that it is not appropriate for direct cell printing due to the irreversible damage that high temperatures and unfavorable pH environments will bring to biological molecules. In order to overcome these problems, researchers are committed to developing new printing ink and printing process, and strive to apply the FDM process for bioprinting at room temperature or lower temperature. Hsieh et al synthesized two thermos-responsive waterborne PU dispersions, which can form a gel at about 37 °C without any crosslinker [193]. They mixed NSCs in the PU dispersion, kept it at 37 °C, and used FDM technology to prepare thermosensitive NSC-laden PU scaffolds, which were composed of eight layers of fibers without severe collapse. The NSCs in 25%–30% PU2 hydrogel possessed good proliferation and differentiation ability, which may be related to the stiffness of hydrogel. The function of adult zebra fish with brain damage was restored following the implantation of a 3D printed NSC-loaded 25% PU2 scaffold. These newly created bio-ink and printing techniques offer fresh perspectives on how to use FDM bioprinting for neural tissue engineering. Wang et al printed a unique double-layer porous conduit consisting of PU/collagen using the double-nozzle low-temperature deposition manufacturing method [194]. The oriented inner collagen layer had micro-pores and the outer PU layer had micro-pores, which respectively played roles of permitting nutrient infiltration and preventing fibrous tissue infiltration. The bilayer guide conduits were implanted to bridge a 10 mm long rat peroneal nerve defect and showed better never repair than the pure PU conduit. These in vitro and in vivo research results indicate that the FDM printing process has potential applications in the field of peripheral nerve regeneration.
4.2.2. Direct ink writing.
Direct ink writing is also a printing method based on extrusion, which is usually executed using the bioplotter system. Unlike FDM, the extrusion head moves in the direction of the x–y–z stage under the control of the computer, while the working platform remains stationary [195]. With this printing technique, a pressurized syringe continually extrudes gel-like materials from a viscous liquid media that may or may not contain cells, and the obtained hydrogel material can be cured with cross-linking agents or UV radiation. 3D-bioplotted scaffolds often have a very smooth surface and a single performance as a result of the extrusion and curing processes. To improve the influence of scaffolds on cell behaviors and function, it is necessary to modify the bioplotted scaffold. Moreover, materials currently available for direct ink writing usually do not have the mechanical properties suitable for implantation. Jakus et al mixed majority graphene and minority PLG as ink, and obtained conductive composite scaffolds with high mechanical strength, good flexibility, and conductivity greater than 800 S m−1 by 3D bioplotting method [196]. Studies conducted in vitro revealed that the composite scaffold enabled human MSCs to adhere, proliferate, and differentiate into neurons while also significantly upregulating the expression of genes associated with glia and neurons.
4.2.3. MEW technology.
The theoretical basis of MEW is electrohydrodynamic (EHD), which refers to the study of the dynamics of electrically charged fluids [197]. In some research reports, MEW was also called near-field electrostatic printing (NFEP) or EHD-jet printing [198, 199]. Under the action of a high voltage electric field, the hemispherical droplets at the nozzle tip become conical (called Taylor cone), and then form a liquid jet when the electric field force overcomes the surface tension of the droplet [200]. This process is similar to electrospinning, except that the receiving distance of MEW is smaller, the charged jet experiences a shorter flight process, and 3D scaffolds with controllable geometry and structure can be created according to the pre-designed data model. Vijayawenkataraman et al used EHD-jet printing to prepare PCL/PPy nerve conduits [201]. With the addition of conductive copolymer (PPy-b-PCL), the mechanical properties of the scaffold were closer to the softness of the natural peripheral nerve, and the conductivity was significantly increased. In vitro cell studies showed that human ESC-derived neural crest stem cells (NCSCs) could adhere, grow and differentiate into neurons on 3D-printed PCL/PPy scaffolds. In another work, they printed PCL/poly(acrylic acid) (PAA) scaffolds with tunable mechanical properties and degradation rate, which possessed mimic mechanical properties (to the native human peripheral nerve), matched conductivity (to the amphibian motor nerve fiber myelin sheath), as well as injury- and site-specific biodegradability (according to the PCL/PAA concentration ratio) [202]. In vivo experimental studies showed that these conductive porous conduits can facilitate nerve excitation and conduction, indicating that EHD-jet 3D printed conductive scaffolds have potential clinical value for peripheral nerve regeneration.
4.3. SLA technology
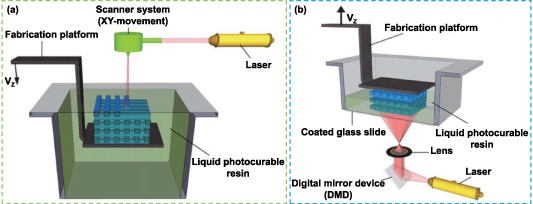
The principle of liquid-photosensitive resin photopolymerization under the influence of ultraviolet (UV) or other light sources is the foundation of the SLA process, which refers to the photopolymerization and solidification of liquid-photosensitive resin [203]. The liquid will harden from point to surface in the area where the computer-controlled laser beam scans the surface of liquid resin while being deflected by a mirror. SLA is a fairly slow printing technique because when the first layer of scanning is finished, the lift drives the platform down one layer and then scans the following layer until a complete 3D solid structure is formed (figure 5(a)) [180]. Tissue engineering scaffolds with clearly defined microstructures and interconnected pores have been created using this printing technique. Although SLA has a higher printing resolution (a few tenths of microns) [204], the resins available for this method are primarily polymers with acrylate, urethane acrylate, or vinyl ether functional groups, which will harm the functionality of the printing scaffold and cause problems with cell adhesion, such as acrylate hydrogels [205]. Surface modification with bioactive substances is typically necessary to improve cell adherence. Zhou et al used dopamine (DA, an essential neurotransmitter) to functionalize GelMA, designed ink that can enhance NSC differentiation, and fabricated GelMA−DA scaffolds with highly porous and interconnected structure using SLA technology [206]. The NSCs cultured on the scaffold showed obvious neural network formation, and enhanced expression of neuron genes.
Figure 5. Schematic representation of (a) stereolithography and (b) surface project technology. Reprinted from [207], Copyright (2012), with permission from Elsevier.
Download figure:
Standard image High-resolution imageCompared with inkjet and extrusion-based printing, SLA does not have the problem of nozzle blocking, so the ranges of bio-ink viscosity and cell density are expanded. Cell viability will be significantly impacted by the basic requirement of SLA, which is that bio-ink should be compatible with the light source [208]. In addition, in the SLA process, the material toxicity caused by the incorporated photo-initiators for cross-linking hydrogels is also one of the main challenges of this technology applied in bioprinting. More studies into biocompatible printing ink are required to improve the application of SLA in clinically relevant neural tissue engineering constructions.
4.4. Projection-based printing
Projection-based printing is also based on the photopolymerization of resin materials [209, 210]. Unlike the bottom-to-up method of SLA, projection-based printing is a top-to-bottom method, which is conducted through a digital light processing (DLP)-printer. The main difference between surface project technology and SLA process is that SLA can only project the light spot of the light beam, while the digital micro-mirror device composed of millions of mirrors in the DLP system can directly project two-dimensional (2D) plane images onto the surface of photosensitive materials, thereby greatly improving the printing efficiency (figure 5(b)) [207]. Although the surface project has many advantages, the printing materials suitable for nerve tissue engineering still need to be developed. Dilla et al synthesized PEG–poly(propylene fumarate) (PPF) and PEG–poly(propylene maleate) (PPM) copolymer with monomethyl ether PEG and PEG–diol as initiators [211]. The breaking elongation of DLP-printed hydrogel was greatly increased. In addition, in vitro cell studies showed that PPF–PEG–PPF triblock hydrogels were biocompatible with SCs, and were expected to be applied in neural tissue engineering. Hydrogel printed with DLP had significantly more breaking elongation. Additionally, PPF–PEG–PPF triblock hydrogels were found to be biocompatible with SCs in cell studies and were anticipated to be applied in the engineering of nerve tissue.
4.5. Emerging 3D printing
4.5.1. Two-photon polymerization (TPP) method.
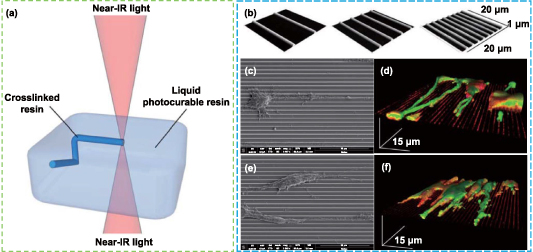
TPP technology is a new 3D printing process based on photopolymerization developed by Kawata et al at the beginning of the 21st century [212, 213]. TPP refers to the photopolymerization process initiated by two-photon absorption (one molecule of a matter absorbs two photons at the same time), which only occurs at the high-intensity laser focal point produced by the near-infrared femtosecond pulsed laser beam. Two-photon absorption does not happen in the optical path outside of the focus (figure 6(a)). As a result, TPP technology, in contrast to the conventional SLA method, overcomes the constraints of the plane surface and may directly conduct photopolymerization at any point in the 3D space [214, 215]. In addition, the most remarkable feature of this technology is that its solidification resolution could be smaller than the diffraction limit of the laser, enabling 3D printing to achieve micro- or even nano-scale, and its horizontal spatial resolution can reach 80 nm [216]. While low printing speed may make it impossible to use high-resolution printing on a large scale in industrial manufacturing, it is appropriate for disciplines that demand more complex structural requirements, such as tissue engineering [217].
Figure 6. Two-photon photopolymerization (TPP) method and its application in nerve repair. (a) The principle of TPP. Reprinted from [207], Copyright (2012), with permission from Elsevier. (b)–(f) The patterned substrates with different frequencies of submicrometric ridges fabricated by TPP, and the behaviors of PC12 (above) and SH-SY5Y cells (below) on the patterned scaffolds. Reprinted with permission from [220]. Copyright (2013) American Chemical Society.
Download figure:
Standard image High-resolution imageKoroleva et al prepared PLA scaffolds with well-defined microstructures by combining TPP, soft lithography, and micro-molding, which greatly improved the production efficiency while achieving high resolution [218]. First, TPP was applied to photopolymerize PLA and rapidly create a high-precision prototype structure, then, soft lithography technology was used to stamp the prototype scaffold to obtain the PDMS mold, and finally, a large number of scaffolds similar to the prototype were fabricated by replicating the PDMS mold through micro-molding technology. The TPP process took about 3 ∼ 5 h, while the micro-molding process took only 10 min. The results showed that the 3D scaffold provided a suitable matrix for the adhesion of SCs, as well as the cells displayed bi-polar and tri-polar morphologies and formed focal contacts, indicating the scaffold could be used for peripheral nerve tissue engineering. Accardo et al developed a 3D free-standing PEGDA scaffold using the TPP method [219]. This 3D scaffold, which has a really distinct structure, may be able to promote neuron2A growth and proliferation, as well as trigger the production of neuron markers and direct neurite extension. Marino et al used the TPP approach to create a submicrometric patterned scaffold, and they next investigated how the scaffold affected cell morphology (figures 6(b)–(f)) [220]. The findings demonstrated that PC12 cells and human SH-SY5Y-derived neurons developed on the parallel-arranged submicron ridges, resulting in closely-arranged neurites of clearly discernible length. These findings suggest a fresh approach for future study on 3D printing technology in the area of nerve regeneration.
4.5.2. Microfluidic printing.
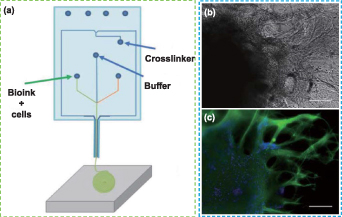
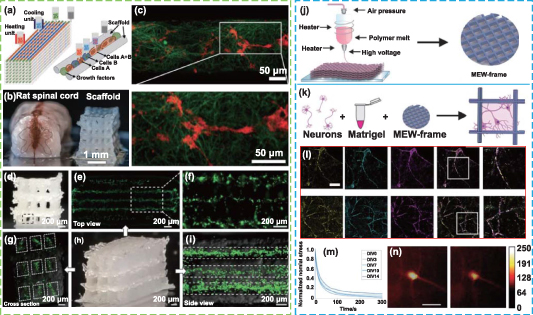
The 3D bioprinting technology based on extrusion is the main strategy for printing nerve tissue constructs [221, 222]. The shear stress that cells experience during printing, which results in reduced cell viability and a lack of long-term tissue functionality, is one of the major obstacles to using these technologies, however [223]. The Lab-on-a-Printer™ technology applies microfluidic channels to 3D printing technology (figure 7(a)), allowing materials to be pretreated and printing settings to be programmed in advance to achieve desired compositions in the printed inks [224]. This microfluidic bioprinting technology offers a preferable technique for printing 3D scaffolds with cell-filled bio-inks that may produce complex and heterogeneous structures with various material components and cell kinds in an accurate manner [225]. This makes it possible for bioprinting precisely designed 3D scaffolds with micro features and macro architectures, and evenly dispersed bio-ink. Abelseth et al applied microfluidic bioprinting technology to build fibrin-based 3D structures, which embedded neural aggregates derived from iPSCs [226]. The cells maintained good viability throughout the printing process and subsequent in vitro culture (up to 41 d), exhibited obvious neurite outgrowth, and expressed neuron markers (figures 7(b) and (c)). In another work, they created a fibrin-based scaffold that encapsulated guggulsterone-releasing microspheres and neural progenitor cells (NPCs) to induce specific neuronal differentiation using the same method [227]. The sustained release of guggulsterone from the microspheres promoted the differentiation of NPCs into dopaminergic neurons after 30 d of culture. To drive specific differentiation of the spinal cord, their research team also fabricated fiber-based nerve scaffolds loaded with NPCs, and exposed them to different small molecules to induce NPCs to differentiate into MNs [228]. Compared with other bioprinting methods, this microfluidic bioprinting process maintained a high level of cell survival (>81%) and differentiation ability. The results showed that the printed nerve construct expressed neuronal markers and other markers related to spinal cord MNs after 15 d of culture, as well as mature MN markers after 30 d of culture, indicating that the microfluidic bioprinting method can be used for high-throughput production of mature nerve tissue similar to the natural spinal cord, which also can be applied in drug screening related to SCI. These studies provide an idea for integrating biological cues into a 3D-bioprinted scaffold to investigate the key driving factors of neuron differentiation and better imitate the diversity of cells in the nervous system.
Figure 7. Microfluidic printing and its application in nerve repair. (a) Schematic representation of microfluidic printing. Reproduced from [228]. CC BY 4.0. (b), (c) Representative images of (b) phase contrast and (c) immunofluorescence of the 3D printed neural aggregates after culture in the scaffold for 41 d. Scale bars in (b), (c): 200 μm. Reproduced from [227]. CC BY 4.0.
Download figure:
Standard image High-resolution image4.5.3. Kenzan method.
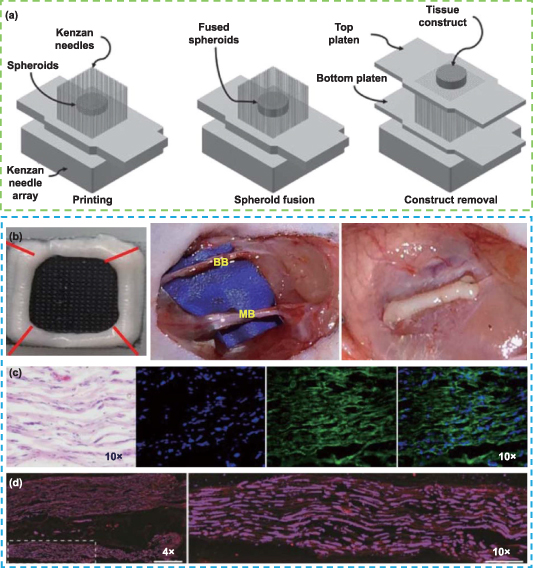
Cells can be assembled directly utilizing the Kenzan method without the use of additional materials [229]. Cell aggregates of a single or a combination of cell types are first cultivated in vitro, and then they are arranged into spherical shapes on a grid of fine needles in accordance with a 3D model that has already been created. To create tightly packed 3D structures, adjacent cell spheres must make contact with one another. These shapes are then transferred to bioreactors and cultivated to produce perfect tissues (figure 8(a)) [230]. The Kenzan method is also known as scaffold-free bioprinting since it relies on intercellular contact to support the 3D structure rather than the hydrogel and other materials used in conventional 3D printing [231, 232]. Zhang et al showed that gingiva-derived MSCs (GMSCs) tended to converge into 3D spheroids, and compared with adherent cells, spherical GMSCs were more likely to differentiate into aligned neuron- and Schwann-like cells [233]. They used the Regenova printing system to create GMSC aggregates that were matured in bioreactors. The organization of the nerve fibers in the GMSC nerve construct was demonstrated in in vivo studies, and the recovery of CMCP was comparable to that of the autograft group, suggesting that the 3D-bioprinted scaffold-free nerve construct promoted regeneration and function recovery of facial nerve injury in rats (figures 8(b)–(d)). This work highlights a new approach for creating nerve tissue constructs.
Figure 8. Kenzan method and its application in nerve repair. (a) Schematic illustration of the Kenzan method and the Kenzan needle are arranged in an array pattern of 26 × 26. Reprinted from [230], Copyright (2019), with permission from Elsevier. (b)–(d) 3D structure of the bioprinting nerve constructs from pure GMSC spheroids, which were transplanted for the regeneration of 5 mm facial nerve defects, and the histological analysis of newly regenerated nerves in the 3D-bioprinted construct implantation. Hematoxylin and Eosin (H&E) staining showed the regenerated nerves. Immunohistochemistry showed the organized axon alignment (stained with β-tubulin III and DAPI), and matured axon and myelin (stained with SMI31/32 and Fluoromyelin). Reproduced from [233]. CC BY 4.0.
Download figure:
Standard image High-resolution image5. The application of 3D printing for peripheral nerve and spinal cord injuries
Nerve injuries lead to the interruption of the nerve network and information transmission throughout the body, which will eventually lead to the loss of motor and sensory function [223, 234]. Unfortunately, there are few effective treatment options due to a number of challenges, including low cell viability and a lack of regeneration cues, an inability to accurately replicate the mechanical and chemical environment of the injured site, and difficulties in successfully delivering cells to the damaged tissue. 3D printing can solve these challenges in a variety of ways: (1) the properties of bio-ink can be designed and adjusted to mimic the specific ECM microenvironment, (2) the scaffold can encapsulate and transport cells to the injured site without adverse effects on cell viability and activity, and (3) 3D-printed scaffold can load and continuously release small molecules or proteins to enhance tissue regeneration. In this chapter, we will discuss the advance of using 3D printing constructs to promote nerve repair after PNI and SCI.
5.1. 3D printing functional scaffolds for guiding nerve differentiation
As a key element in tissue engineering, scaffold materials not only play a role of structure support, but also can guide cell differentiation to determine its fate. Therefore, it is particularly important to design scaffold materials reasonably to simulate the physical/chemical environment and structure characteristics of natural nerve tissue. In the process of 3D printing to create the nerve scaffold, the physical and biochemical properties of printing ink and the structure of the formed scaffold can be pre-designed to obtain a functional scaffold that can guide nerve differentiation. Rajaram et al printed sodium alginate/HA/SCs using the bioplotting method [235]. For cross-linking, each scaffold was submerged in a calcium chloride solution, which might keep the scaffold's structure and the activity of SCs intact. The sodium alginate/HA porous scaffold was appropriate for neural tissue engineering because it had good structural integrity and sustained cell activity. Although 3D bioplotting can produce cell-integrated biomaterials quickly, more attention must be paid to developing durable and extrudable printing materials with desirable mechanical and physical characteristics. Huang et al blended graphene or graphene oxide with biodegradable waterborne PU to fabricate graphene-based nanocomposite hydrogel for NSC printing (figures 9(a)–(e)) [236]. The composite hydrogel ink's rheological characteristics made them acceptable for 3D printing and NSC survival. Additionally, the relatively modest concentration of graphene (25 ppm) added to the printed scaffolds dramatically promoted neuronal differentiation of NSCs and oxygen metabolism. These works provide a reference for the design of bio-ink for the 3D printing of nerve tissue. Lee et al combined SLA technology and coaxial electrospraying to fabricate 3D poly(ethylene glycol)/poly(ethylene glycol) diacrylate (PEG/PEGDA) composite hydrogel scaffolds with core–shell nanoparticles that can continuously release neurogenic factor [237]. The results showed that incorporated NGF-loaded nanoparticles increased the surface roughness of the scaffolds and promoted neural differentiation of PC12 cells. Meanwhile, sustained released NGF promoted the average neurite length of primary neurons. 3D-printed scaffolds can guide PC12 cells to extend neurites along the fiber direction, indicating the potential of this scaffold in improving nerve cell function. In their another work, highly oriented electrospun fibers were incorporated into PEG-based scaffolds printed by SLA to enhance mechanical properties and biocompatibility [238]. In vitro studies showed that the primary cortical neurons cultured on the scaffold displayed neurite outgrowth, and neurites extended through the isolated pores of the 3D-printed scaffold. The success of in vitro studies has encouraged many researchers to evaluate the feasibility of 3D bioprinting scaffold in vivo as an alternative therapy for PNI. In a recent study, Wu et al bioprinted the gelatin/alginate hydrogel scaffolds loaded with SCs (figures 9(f)–(h)) [239]. The results of in vitro cell culture showed that SCs had high viability and could adhere well to the surface of the scaffold. The cells in the 3D bioprinting scaffold released higher levels of NGF than the 2D culture group. In addition, after 4 d of culture, the mRNA levels of NGF, BDNF, GDNF, and platelet-derived growth factor expressed by SCs in 3D scaffolds were higher than those in 2D culture. Four weeks after implantation of the PNI model in mice, the cell-laden scaffolds still displayed partial lattice structure, positive S100β immunofluorescence, and few inflammatory responses. These results showed that 3D-bioprinted gelatin/alginate/SCs composite scaffolds improved the expression of cell adhesion and related factors, and proved the consistency and correlation between in vitro release behaviors and in vivo studies for neurotrophic factors. These works highlight the importance of simulating the biochemical environment and structural features of natural tissues, and the potential application of 3D printing technology in promoting nerve regeneration and repair.
Figure 9. 3D-bioprinted scaffolds and their applications in nerve differentiation. (a)–(e) The photos of a 3D-bioplotted neural construct loaded with NSCs, and the oxygen metabolism (after 24 h) and neural-related gene expressions (after 72 h) of NSCs in different hydrogel constructs. Reproduced from [236] with permission from the Royal Society of Chemistry. G-P refers to pluronic-modified graphene, and OCR refers to oxygen consumption rate. (f)–(h) The structure of 3D-bioprinted gelatin/sodium alginate scaffold, and the results of in vitro and in vivo studies. The arrows indicate channels inside the 3D scaffold (f) and pseudopodia from the Schwann cell (g). Scale bar in (h): 50 μm. Reprinted from [239], Copyright (2020), with permission from Elsevier.
Download figure:
Standard image High-resolution imageTaking into account the electrophysiological characteristics of nerve tissue, some studies also used 3D printing to prepare conductive scaffolds. Weng et al successfully printed conductive PPy/collaborative composite scaffolds using inkjet technology (figures 10(a)–(c)) [240]. They patterned PPy and collagen on the polyacrylate film to impose spatially controllable ES. PPy/collagen scaffolds enhanced the attachment and growth of PC12 cells, and increased the neurite outgrowth and orientation under ES. In order to produce conductive nerve scaffolds, Housthyar et al used extrusion-based printing to develop a new nanocomposite ink made of DA, carbon nanofibers, and PCL (figures 10(d)–(f)) [241]. The mechanical properties of printed PCL membranes are 30% better than those of pure PCL membranes and are comparable to those of a natural neuron. The print line provided support for the adhesion, migration, and differentiation of nerve cells, according to in vitro cell studies. This suggests that the developed scaffold could be used for nerve guidance based on its signal transmission and guiding neurons in the proper pathway toward the target site. Heo et al prepared conductive PEDOT: polystyrene sulfonate (PSS) nanofibers, and mixed them with PEGDA to obtain a photocurable prepolymer that was printed by SLA technology to construct a patterned conductive hydrogel scaffold (figures 10(g)–(j)) [242]. With the increase of PEDOT: PSS concentration, the conductivity of the hydrogel increased significantly. The patterned conductive scaffolds can not only provide structural support, but also transfer the ES toward the dorsal root ganglion (DRG) cells grown on the scaffold, resulting in enhanced neuronal differentiation. Similarly, Lee et al fabricated conductive MWCNT/PEGDA nerve scaffolds with intricate microarchitectures [243]. The results showed that the incorporation of MWCNT facilitated the proliferation and early neuronal differentiation of NSCs. In addition, the ES could have a synergistic effect on promoted neuronal maturity, indicating 3D printing of MWCNT/PEGDA scaffolds was an effective strategy for nerve regeneration. Additionally, Zhu et al integrated SLA technology and low-level laser therapy (LLLT) to improve the interaction between nerve cells and scaffolds (figures 10(k)–(m)) [23]. It was reported that LLLT could stimulate the photoreceptors on mitochondria and cell membranes, so as to convert light energy into chemical energy in the form of adenosine triphosphate in cells, leading to promoted cell proliferation and function [244]. They first printed 3D transparent GelMA/PEGDA hydrogel scaffolds and seeded NSCs on them, then the cells were stimulated with 635 nm low-level laser light. The findings indicated that this kind of light stimulation can encourage NSCs to differentiate into neurons while inhibiting their differentiation into glial cells, indicating the combination of SLA and LLLT may offer a potent remedy for nerve tissue repair.
Figure 10. 3D-printed constructs with electrical/light stimulation and their applications in nerve differentiation. (a)–(c) Schematic presentation of conductive PPy/collagen substrate for PC12 cell culture, and the images of inkjet printed PPy lines and PC12 cells grown on the conductive scaffold. Reprinted from [240], Copyright (2012), with permission from Elsevier. (d)–(f) Schematic illustration of guiding directional growth of neural cells using 3D-printed functionalized nanocomposite ink line, and neural cell behaviors cultured on pure PCL film and the CNF-based ink line, respectively. Cells were stained with DAPI (blue) and rhodamine phalloidin (red). Reproduced from [241] with permission from the Royal Society of Chemistry. (g)–(j) Schematic presentation of the 3D conductive nerve scaffold, and the behavior and function of DRG cells in the 3D-printed constructs with ES: (h) PEDOT/PSS 0.00%, (i) PEDOT/PSS 0.91% and (j) 3D image of PEDOT/PSS 0.91%. Cells were stained with cell nuclei (blue), neurofilament (green), and Tuj1 (red). Reprinted from [242], Copyright (2019), with permission from Elsevier. (k)–(m) Schematic illustration of the 3D-printed scaffold with light stimulation, the porous structure of the scaffold, and the neuron differentiation of NSCs at day 14 with (above) or without (below) light stimulation. Cells were stained with cell nuclei (blue) and Tuj1 (red). Reproduced from [23]. © IOP Publishing Ltd. All rights reserved.
Download figure:
Standard image High-resolution image5.2. 3D printing NGCs for peripheral nerve repair
A large number of studies have proved that NGCs, as a kind of tissue engineering implants, have great application prospects in the repair of PNI. However, the existing NGCs have many challenges, such as complicated manufacturing processes, simple architecture, and lack of effective guidance cues. As a rapid prototyping technology, 3D printing can easily and quickly create the required structure to simulate the macro- and micro-structure of neural tissue. In addition, by improving the printing process and optimizing the printing materials, cells, biological factors, etc can be incorporated into the NGCs to promote axon regeneration. Qian et al created single- or multi-layered graphene and PCL composite scaffolds using inkjet technology (figures 11(a)–(c)) [245]. During the printing process, the LBL casting method was integrated to modify the scaffolds with polydopamine and RGD, and finally, a conductive multi-layered porous scaffold with improved cell adhesion was obtained. These 3D conductive scaffolds can significantly improve neural expression in vitro and in vivo, and promote axon regeneration and remyelination after PNI. In order to achieve simple and rapid manufacturing of NGCs suitable for clinical application, Shim et al designed and prepared poly(caprolactone-co-lactide) (PCLA) scaffolds for peripheral nerve regeneration using 3D bioplotter [246]. Bioplotted PCLA conduits presented flexible characteristics with interconnected pores. Nerve regeneration was investigated after implantation of a 15 mm sciatic nerve defect in rats during 2–16 weeks. The results showed that more and more regenerated nerves grew into the nerve conduits, indicating the conduits had been well integrated into the host damaged nerve. Eight weeks after implantation, the bioplotted nerve conduits remained their original shape without collapse, which matched the nerve regeneration rate, indicating that the conduits met the basic structural support performance. In addition, the regenerated nerve in the conduits gradually grew across the sciatic nerve injury gap and connected from the proximal end to the distal end along the axis of NGC. The histological analysis showed that there were obvious myelinated axons in the bioplotted PCLA conduits. These studies confirmed that 3D printing was a potential method for constructing scaffolds applied in nerve tissue engineering, and the NGCs fabricated by printing technology are feasible for clinical peripheral nerve regeneration.
Figure 11. 3D-printed multi-layered nerve guide conduit (NGC) and its application in peripheral nerve repair. (a) Schematic presentation of graphene nerve conduit construction with inkjet printing, (b) relative gene expressions (GFAP, Tuj1, and S100) of different scaffolds, and (c) TEM images for regenerated axons in different groups after 18 weeks of implantation. Reproduced from [245]. CC BY 4.0.
Download figure:
Standard image High-resolution image5.2.1. Anisotropic NGCs improve the microenvironment of peripheral nerve repair.
As previously mentioned, the peripheral nerve is composed of a series of nerve fiber bundles arranged in parallel. Therefore, by simulating and improving the microenvironment of the damaged peripheral nerve, the cell behavior and function in the injured site can be enhanced, finally promoting nerve tissue repair. Li et al constructed a 3D printing scaffold loaded with NCSC-derived SC generators (NSCPs) for peripheral nerve repair (figures 12(a)–(e)) [199]. They prepared multiscale fiber scaffolds with different diameters on the longitudinal and lateral axis using EHD 3D printing, specifically, in the direction along the nerve, 10 µm ultrafine fibers are printed with an interval of 100 µm to induce the directional arrangement of cells and the directional growth of axons, while in the direction perpendicular to the nerve, 60 µm fibers are printed with an interval of 500 µm to support the entire structure and maintain the scaffold morphology. In vitro studies showed that NCSCs cultured on the 3D fiber scaffolds can well proliferate, migrate, extend, and successfully differentiate into SC precursors. The cells grown on the longitudinal thin fibers showed more extension behavior, forming a structure similar to bands of Büngner, while the cells grown on the lateral fibers gathered in large numbers, providing a repository for cell migration. 3D-printed scaffolds loaded with NSCPs were implanted into the rat sciatic nerve defect model, which promoted the directional growth of axons and the formation of the myelin sheath. After 6 months, the scaffolds were basically degraded, and the density of myelinated nerves increased, showing an excellent repair effect. In order to explain the mechanical theory of topological cues of scaffold materials in promoting peripheral nerve regeneration, Dong et al used MEW and electrospinning technology to construct a nerve conduit with a core–shell structure consisting of aligned microfiber-bundle cores and randomly ordered nanofiber sheaths [247]. Studies have shown that macrophage recruitment and subsequent polarization toward a pro-healing phenotype may be encouraged by aligned microfibers in the conduit, which in turn enhanced SC migration, myelination, and axon extension. The results of in vivo study also proved that the nerve conduit with core–shell structure significantly improved the regeneration of the injured sciatic nerve in rats. This work provides an important insight into the regulation of topological cues in scaffold materials on functional nerve regeneration through immune mechanisms. Zhang et al made full use of the interdisciplinary characteristics to design NGC from three aspects: anisotropy, photocatalytic stimulation, and self-assembly at the implant site (figures 12(f)–(m)) [248]. First, the researchers constructed the anisotropic PCL microfiber structure by MEW, and realized the directional axon extension of PC12 cells. Secondly, the functionalization of PCL micropattern with GO and graphitic carbon nitride realized photoelectric conversion and wireless ES, which further enhanced the extension length of neurites with visible light stimulation. Finally, NGC was successfully fabricated by manual rolling or self-assembly of the thermal responsive two-layer system. These studies highlight the synergy of multiple factors in the process of peripheral nerve repair and the potential application of MEW technology in creating ideal NGCs, and provide new ideas for tissue engineering to treat PNI.
Figure 12. Anisotropic NGCs and their applications in peripheral nerve repair. (a)–(e) Schematic diagram of 3D printed multi-scale NGC mimicking the microenvironment after PNI, architectures of the bionic scaffold and NGC, and in vitro and in vivo study for peripheral nerve regeneration. From left to right in (e): epineurium, NSCPs, scaffold, N + S, normal. Scale bars in (b): 500 µm; in (c): (i) 200 µm, (ii) 100 µm, and (iii) 10 µm; in (d): (i) 100 µm and (ii), (iii) 50 µm; in (e): 100 µm. [199] John Wiley & Sons. [© 2021 Wiley-VCH GmbH]. (f)–(m) Schematic illustration of 3D bioactive NGC functionalized with visible-light photocatalyst, anisotropic architectures of the 3D-printed scaffold and NGC, and guiding effect on behaviors of PC12 cells. Cells were stained with Hoechst (blue) and TUJ1 (red). Scale bars in (g): 100 μm; in (i): 1 mm; in (j): 100 μm; in (k): 20 μm; in (l): 1 mm. Reprinted from [248] Copyright (2020), with permission from Elsevier.
Download figure:
Standard image High-resolution image5.2.2. Drug delivery NGCs enhanced peripheral nerve regeneration.
Drugs used to treat damaged nerves can also be loaded in nerve scaffolds in addition to cells and bioactive substances. Some medications have recently been placed within nanoparticle carriers and then inserted into 3D-printed scaffolds. This procedure prolonged the time of drug release while simultaneously ensuring the quality and efficacy of the pharmaceuticals. In order to create drug-loaded 3D nerve conduits using DLP bioprinting technology, Xu et al loaded RGFP966 (a histone deacetylase 3 inhibitor that negatively regulates chromatin modification by inhibiting the activation of the PI3K–AKT–ERK signaling pathway) into monomethoxy PEG–PCL nanoparticles (figures 13(a)–(e)) [249]. The conduits not only had a macro-designed structure and the inner surface of directional fiber arrangement, but also could continuously release RGFP966 to activate the PI3K–AKT–ERK signal pathway, leading to the development and regeneration of the myelin sheath. Its ability to induce nerve regeneration and functional recovery after implantation into the rat sciatic nerve defect model was comparable to that of an autologous graft. This research team's efforts to increase the high expression of yes-associated proteins (YAP) in the nucleus, which in turn promotes the interaction between YAP and the myelin gene promoter and facilitates the extension of the myelin sheath, were similar to how they encapsulated XMU-MP-1 (a Hippo pathway inhibitor). (figures 13(f)–(j)) [24]. Also, DLP technology was used to prepare nanoparticle-enhanced nerve constraints composed of GelMA hydrogel with drug-loaded PEG–PCL nanoparticles. The 3D-printed drug releasable conduit provided a microenvironment for axon extension, and SC proliferation and migration. The nanoparticles encapsulated in the scaffold continuously released drugs to promote nerve regeneration and ultimately effectively enhanced the functional recovery of sciatic nerve injury, indicating the potential clinical value of the scaffold in peripheral nerve repair. This drug-loaded 3D printing technique provides a large development and exploration space and can be used for numerous related therapeutic compounds that affect peripheral nerve regeneration. Recently, they also created a platelet-incorporated hydrogel conduit [250]. The findings demonstrated that the hydrogel could considerably extend platelet survival, and that the live platelets in the conduit could continually release a variety of growth factors to aid in nerve healing. The conduit's successful use to close a 10 mm sciatic nerve gap demonstrated its high therapeutic efficacy, suggesting that 3D-bioprinted nerve conduit with live platelets may have clinical uses in peripheral nerve repair.
Figure 13. Drug delivery NGCs and their applications in peripheral nerve regeneration. (a)–(e) Schematic illustration of 3D-bioprinted nerve conduit modified with RGFP966 laden nanoparticles (R-NPs), the structure of NGC with R-NPs, and the efficacy of 3D printed conduit with R-NPs (3DRC) and 3D printed conduit (3DC) in vivo after 3 months of surgery. Scale bars in (c): 200 µm; in (d): 200 µm; in (e): 1 µm. Reprinted from [249] Copyright (2019), with permission from Elsevier. (f)–(j) Schematic presentation of the 3D printed nanoparticle-enhanced NGC, the structure of the 3D printed nerve conduit decorated with XMU-MP-1 loaded nanoparticles, and the efficacy of 3D printed drug releasable conduit (3DDC) and 3D printed conduit (3DC) in vivo after 3 months of implantation. Scale bars in (h): 100 µm; in (i): 10 µm; in (j): 1 µm. Reprinted from [24], Copyright (2019), with permission from Elsevier. H&E staining exhibited the transverse sections of the nerves, Luxol Fast Blue (LFB) staining displayed the morphological change in the regenerated nerve fibers, and TEM images showed regenerated myelinated axons.
Download figure:
Standard image High-resolution image5.2.3. Individualized NGCs accelerate the clinical practice of peripheral nerve repair.
To verify the clinical application of SLA technology in the field of nerve repair, Pateman et al used micro-SLA to prepare PEGDA-based nerve scaffolds for personalized peripheral nerve repair [251]. In vitro studies showed that rat DRG explants cultured on the 3D-printed scaffolds displayed ganglion body adhesion, neurite formation, and SC migration. The customized NGCs were implanted into the mouse common fibular nerve injury model, and were able to support re-innervation across the 3 mm injury gap after 21 d, overall, the repair effect was similar to that of the autograft group. Ye et al optimized printing parameters and fabricated multichannel NGCs with different inner diameters using the DLP method (figures 14(a)–(f)) [252]. In vitro co-culturing of PC12 cells and NCSCs showed that nerve cells could proliferate and migrate along the longitudinal channel of the scaffold, and NCSCs were induced to differentiate into neurons, indicating that DLP technology can be used to rapidly and accurately manufacture NGCs for nerve repair. Zhu et al developed a rapid continuous 3D printing method to create NGCs with customized geometry and complex architectures (figures 14(g)–(m)) [253]. By changing the printing conditions of DLP (such as the photoinitiator concentration and the light-exposure intensity), a series of GelMA-PEGDA scaffolds with adjustable mechanical properties (Young's modulus of 0.3 ∼ 4.5 MPa) were prepared. In addition, they implanted 3D-printed GelMA-PEGDA conduits with four microchannels into the transected sciatic nerve gap of mice. The usefulness of nerve conduits for the directional guidance of regenerated nerve was demonstrated after 11 weeks of implantation when the regenerated nerve split into four branches at the proximal end and united into a nerve at the distal end. All these studies emphasize the potential of 3D printing in rapidly producing accurate and complex NGC, especially allowing individual customization.
Figure 14. Individualized NGCs and their applications in peripheral nerve repair. (a)–(f) The structure of DLP printed NGCs with multiple channels, and effects on differentiation and functions of NCSCs. Cells were stained with EGFP (green), Tuj1 (blue), and PGP9.5 (red). Reprinted from [252], Copyright (2020), with permission from Elsevier. (g)–(m) 3D printed NGCs with individual customization, and in vivo study for sciatic nerve injury. H&E staining in (m) showed the regenerated nerve and NGCs at different sites after 11 weeks of implantation. Scale bars in (m): 200 mm (top row) and 100 mm (bottom row). Reprinted from [253], Copyright (2018), with permission from Elsevier.
Download figure:
Standard image High-resolution image5.2.4. Specially designed NGCs facilitate peripheral nerve repair.
NGCs can not only directly guide peripheral nerve regeneration, but also serve as an effective auxiliary tool to assist neurorrhaphy to facilitate the clinical treatment of PNI. Zhang et al designed a self-adhesive drug-loaded bandage that can form a wrap around the injured nerve to promote nerve regeneration and recovery (figures 15(a)–(g)) [254]. The bandage is realized by constructing two different hydrogel layers through a DLP printer, one is a rectangle layer composed of dibenzyl cyclooctyne modified (DBCO-) GelMA, and the other is a grating layer composed of azide modified (N3−) GelMA loaded with XMU-MP-1 nanoparticles. By the click reaction of −N3 and -DBCO, the bandage possessed an adhesion effect, making the use of the bandage simple and efficient. The medications in the bandage were primarily delivered to the inner portion of the wrap due to the grating layer's proximity to the wounded area. This improved SC proliferation and migration, which in turn encouraged nerve regeneration. The findings demonstrated that the bandage may be used to aid neurorrhaphy, that its self-adhesiveness could facilitate installation without additional suturing, and that bandages laced with drugs could aid in the healing of damaged nerves. Tao et al fabricated a shape-memory conduit made of gelatin cryogels through indirect 3D printing technology with 3D-printed moulds (figures 15(h)–(m)) [255]. The conduit has outstanding mechanical qualities and a porous construction. It was susceptible to mechanical force-induced collapse, but it was also capable of recovering its former shape after absorbing physiological saline. The conduit's ability to remember shapes makes installation easier during surgery. This biodegradable conduit has potential clinical uses in nerve regeneration as evidenced by in vivo studies showing that it may wrap the sciatic nerves of rats following neurorrhaphy and support the functional recovery of the transected peripheral nerve.
Figure 15. Specially designed NGCs and their applications in peripheral nerve repair. (a)–(g) Schematic illustration of the self-adhesive drug-loaded bandage (SADB), the structure of 3D printed SADB, and study of in vitro drug release and in vivo nerve repair. Images of light microscope (e) and TEM (f) showed regenerated axon and myelin sheath, and H&E staining in (g) showed the muscles. Scale bars in (b): 5 mm; in (c): 200 µm; in (e)–(g): 50 µm. Reproduced from [254]. CC BY 4.0. (h)–(m) Schematic presentation of the NGC with shape-memory after end-to-end neurorrhaphy, and the morphologies of the conduit in different stages during the deformation, and in vivo study for sciatic nerve injury. From left to right in (i): Original, after deformation, and after inflation. (j and l) Conduit groups and (k and m) end-to-end groups were shown. Reproduced from [255]. CC BY 4.0.
Download figure:
Standard image High-resolution image5.3. 3D printing nerve construct promotes the establishment of the spinal cord network
The precise spatial arrangement of cells is crucial for the functional growth of tissues, which is particularly important for the spinal cord because it contains a series of cell types with various organizational forms. Joung et al employed extrusion-based bioprinting technology to create a biocompatible scaffold with precisely positioned cells to mimic the organizational characteristics of particular cell types in the spinal cord (figures 16(a)–(i)) [256]. This is achieved by repeatedly printing a base layer first, then printing 150 µm wide channels, and accurately point-distributing a cluster of cells consisting of spinal NPCs (sNPCs) with or without oligodendrocyte progenitor cells (OPCs) in the channels. The results confirmed the viability of cells after printing, and the fact that sNPCs differentiated and generated axons that extended throughout microscale scaffold channels and formed functional neural networks. In addition, co-printing OPCs and sNPCs can make these two types of cells form a closer physical connection, thus promoting the reconstruction of functional axon connections across the injured spinal cord tissue. This study highlighted the feasibility of constructing multicellular neural tissue with the precise spatial organization, which could promote the establishment of the spinal cord network. Gu et al printed hNSCs-laden composite hydrogel ink to construct neural mini-tissue in lattice shapes [257]. The bio-ink was composed of alginate, carboxymethyl-chitosan, and agarose. The stem cells in situ proliferated and differentiated into GABAergic neurons and neuroglia cells after bioprinting. In addition, differentiated neurons formed neurite contacts, established nerve networks, and showed increased bicuculline-induced calcium response, indicating the effectiveness of the hydrogel platform to simulate the physical and chemical interactions between nerve cells and their microenvironment, which can be used to create functional 3D neural tissue. Although these current works were performed in vitro, recapitulating the heterogeneous nature with the complex multicellular architecture of the spinal cord may be critical for functional regeneration of the spinal cord in vivo.
Figure 16. 3D printed constructs and their applications in the establishment of spinal cord network. (a)–(i) 3D printed biocompatible spinal cord scaffolds with sNPCs and OPCs, and in vitro study for SCI. OPCs and sNPCs expressed mCherry (red) and β3III-tubulin (green, a marker of the neuron) in (c). [256] John Wiley & Sons. [© 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim]. (j)–(n) Schematic illustration of 3D-printed construct, and in vitro study for spinal cord network. Immunocytochemical staining in (l) showed protein expression of inhibitory synapse in 3D primary spinal cord culture after 14 and 21 d, and cells were stained with VGAT (yellow), Gephyrin (cyan), and GlyR (magenta). Mechanical testing in (m) referred to 3D primary spinal cord after culture of 0, 3, 7, 10, and 14 d. Calcium imaging in (n) corresponded to neuronal activity of the spinal cord after a culture of 14 d. Scale bar in (l): 50 μm; in (n): 50 μm. [25] John Wiley & Sons. [© 2021 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH].
Download figure:
Standard image High-resolution imagePrecise spatiotemporal morphogen gradients that generate particular gene expression patterns and control the proliferation and differentiation of NPCs are what drive the distinct cell populations with varied functions in the spinal cord [258]. Inkjet printing can be utilized to make macromolecular gradients in bioprinting scaffolds that can stimulate NSC differentiation, according to Ilkhanizadeh et al [259]. To examine their subsequent impacts on primary fetal NSCs, they added fibroblast growth factors-2 (FGF2), CNTF, and fetal bovine serum (FBS) to a polyacrylamide-based bio-ink, respectively. While CNTF and FBS promoted the production of astrocyte markers (GFAP) and smooth muscle markers (smooth muscle actin), respectively, FGF2 did not cause the differentiation of NSCs but continued to maintain the proliferation ability. Then they constructed a scaffold with a gradient of CNTF concentrations, ranging from low to high, and they tracked the gradient of cell differentiation. Particularly, the expression of GFAP increased linearly in the cells exposed to greater concentrations of CNTF. This work showed the significance of growth factors in controlling the activity of nerve cells, despite the fact that these phenomena were not unique to the spinal cord, and also highlighted the inkjet bioprinting method that can be utilized to print bioactive compounds with stable gradients. Fischhaber et al coupled the main spinal cord neurons in the MA with the MEW frames utilizing the microfiber reinforcement approach to better imitate the biological and mechanical environment of cells in the ultrasoft tissues of the spinal cord (figures 16(j)–(n)) [25]. The researchers analyzed the maturation of inhibitory glycinergic synapses through protein expression, complex mechanical characteristics, and calcium imaging, thus evaluating the neuronal network development in the 3D constructs. The results showed that the 3D expression pattern of marker proteins in inhibitory synapses and the mechanical performances were similar to those in natural spinal cord. In addition, the 3D spinal cord neural network showed strong neuronal activity. This work highlights the feasibility of using MEW fiber-reinforced ultrasoft matrix to simulate the culture of spinal cord cells, and provides a favorable tool for further studying the biomechanical mechanism of SCI repair.
5.4. 3D printing spinal cord scaffold for the repair of SCI
A series of reactions occur after SCI, such as a large number of nerve cell death, ischemia, inflammatory reaction, and glial scar formation. 3D printing provides a variety of feasible solutions using distinct bio-ink materials and incorporating distinct components (such as cells, growth factors, and biomolecules) to promote spinal cord regeneration. The bio-ink composed of biomaterials, living cells, and/or bioactive factors can be printed, and the physiological structures mechanical properties and biological functions of tissues or organs can be better simulated by precisely regulating the proportion of each component in the bio-ink and printing conditions, finally achieving accurate manufacturing and rapid repair of injured tissues/organs.
5.4.1. 3D printing scaffold simulating spinal cord anatomy for SCI repair.
Benefit from the high resolution and the capacity to print high cell concentration of surface project technology, Koffler et al created a PEGDA-GelMA scaffold for SCI repair using the microscale continuous projection printing method (figures 17(a)–(f)) [260]. The NPCs- encapsulated scaffolds were implanted into the T3 spinal cord transection of rats. After 4 weeks of implantation, the scaffolds were infiltrated with host axons, and the regenerated axons were myelinated by host SCs, with mild spinal cord inflammation. 6 months after implantation, the host axons formed a functional connection with the grafted cells, that is, the injured host axons regenerated into the scaffold and formed synapses with the grafted NPCs loaded in the scaffold, while the axons of the grafted NPCs in turn extended axons out from the scaffold and into the host spinal cord caudal to the injury, resulting in restored synaptic transmission and improved functional recovery. Furthermore, the scaffolds contributed to the electrophysiological properties, indicating that the signal was transmitted at the lesion site. These results confirmed that the 3D-printed scaffold loaded with NPCs supported axon regeneration and formed 'neural relays' across the complete SCI sites in vivo, which was very beneficial to the repair of SCI. In another study, Jiang et al printed 3D collagen/silk fibroin (3D-CF) scaffolds with cavities to simulate normal spinal cord anatomy (the four cavities correspond to cuneate fasciculus, gracile, corticospinal, as well as spinal cord thalamus tracts respectively), and encapsulated NSCs into the scaffolds to promote spinal cord repair in rats [261]. The neurological scores of the 3D-CF + NSCs group were considerably greater than those of the other groups (sham, SCI, and 3D-CF), including enhanced motor behaviors and superior electrophysiological performances, according to the results of an in vivo investigation. In addition, the 3D-CF + NSCs group outperforms other groups in terms of spinal cord continuity and injury cavity filling, as well as having more regeneration axons and less glial scarring. These investigations stress the significance of restoring the metabolic composition and natural structure of the target nerve tissues.
Figure 17. 3D-printed nerve scaffolds with bionic structure and their applications in SCI repair. (a) Schematic illustration of 3D-printed scaffold mimicking the linear organization of spinal cord, and (b)–(f) in vivo studies of the biomimetic scaffolds laden with NPCs for SCI repair after 6 months of implantation. Host-to-graft in ((c) and (e)), and graft-to-host in ((d) and (f)) revealed the interaction between graft and host. Scale bars in (b): 250 µm; in (c): 50 µm; in (d): 10 µm; in (e): 40 µm; in (f): 5 µm. Reproduced from [260], with permission from Springer Nature.
Download figure:
Standard image High-resolution image5.4.2. 3D printing scaffold simulating spinal cord function for SCI repair.
To enhance the mechanical properties and axon guidance function of the scaffolds implanted in the body after SCI, Silva et al developed a biphasic tubular scaffold with tunable mechanical properties for SCI repair [262]. The scaffold was comprised of a starch/PCL semi-rigid tubular porous structure and the filled gellan gum hydrogel concentric core, and in this system, the former provided structure stability of the whole scaffold through establishing connections to the adjacent vertebral bone, while the latter provided biological cues to support axon regeneration by encapsulating nerve cells. In vitro and in vivo studies showed that this scaffold with biphasic structure processed fully interconnected network of pores and appropriate mechanical properties, could support various behaviors of nerve cells, and could integrate well with the injured spinal cord of the host without serious inflammation, ultimately promoting the repair of SCI. In order to achieve the controlled release of neurotrophic factors, Liu et al developed a collagen/chitosan scaffold integrated with BDNF (3D-CC-BDNF) for the treatment of SCI through low-temperature 3D bioplotting (figures 18(a)–(d)) [263]. Compared with the 3D-printed collagen/chitosan scaffolds adsorbed with BDNF (3D-CC + BDNF), 3D-CC-BDNF prolonged the release of BDNF, and promoted the attachment and proliferation of NSCs and hUCBMSCs. Eight weeks after the implantation of 3D-CC-BDNF into the transected lesion of the T10 spinal cord in rats, the scaffold filled the cavity of the injury site, reduced the formation of the glial scar, accelerated the establishment of neurite connection, enhanced the myelination of regenerated nerve, and significantly improved the motor function of rats. These results indicate that 3D-bioplotted scaffolds may be an alternative clinical treatment for SCI in the future. Chen et al created collagen/heparin sulfate scaffolds loaded with basic FGF (bFGF) through extrusion-based bioprinting at −20 °C (figures 18(e)–(g)) [264]. The research results showed that the incorporation of heparin sulfate significantly enhanced the compression performance of the scaffolds, and also improved the mobilization and absorption of bFGF onto collagen. In addition, the implantation of composite scaffolds at the T10 injury site of the rat spinal cord improved motor functions and electrophysiological functions, as well as facilitated the generation of cell neurofilaments. These works provide a method to continuously release growth factors to promote axon elongation, while the scaffold has mechanical properties similar to those of natural tissues.
Figure 18. 3D-printed nerve scaffolds with bionic function and their applications in SCI repair. (a)–(d) The macrostructure and porous microstructure of 3D-CC-BDNF, and histological analysis for SCI repair after 8 weeks of implantation in different groups. Immunofluorescence staining in (d) indicated the establishment of synaptic connections. Scale bars in (b): 300 µm; in (c): 50 µm; in (d): 500 µm. Reproduced with permission from [263]. Copyright © 2021, Oxford University Press. CC-BY-NC. (e)–(g) 3D-bioprinted collagen/heparin sulfate constructs for enhancing functional recovery after SCI. C/H, 3D-C, and 3D-C/H were the scaffolds fabricated by freeze dry method, 3D-bioprinted collagen scaffolds, and 3D-bioprinted collagen/heparin sulfate scaffolds that had been implanted into the SCI model, respectively. Immunostaining in (g) revealed positive neurofilaments (NF) in different groups. Scale bars in (f)–(g): 50 µm. [264] John Wiley & Sons. [© 2017 Wiley Periodicals, Inc.].
Download figure:
Standard image High-resolution image5.4.3. 3D bioprinting improves stem cell therapy after SCI.
Because of the sensitivity and fragility of NSCs, 3D bioprinting with NSCs has many problems, such as few kinds of selectable bio-ink, cumbersome printing process, low cell viability after printing, and weak interaction of cell-scaffold, which greatly limits its application in SCI treatment. In response to the above challenges, Liu et al constructed a nerve scaffold with a bionic structure of the spinal cord using 3D bioprinting, which provided a good microenvironment for the survival of NSCs and their differentiation into neurons, and was applied to the treatment of SCI in rats (figures 19(a)–(f)) [26]. In this treatment strategy, researchers innovatively designed and prepared the bio-ink with good printability and biocompatibility, which is composed of hydroxypropyl chitosan (HBC), thiolated HA (HA-SH), vinyl sulfonated HA (HA-VS) and MA. Using the excellent temperature response of HBC and the spontaneous secondary cross-linking of Michael addition reaction between HA-SH and HA-VS, the one-step bioprinting facilitated the creation of a living neural scaffold loaded with NSCs. The whole printing process was smooth, the printing strands were cured quickly (gelled within 20 s at 37 °C), the shaped structure was stable, and the NSC viability in the printed scaffold could be as high as 95%. By optimizing the structure and function of the scaffold, the interactions of cell-cell and cell-scaffold were effectively enhanced, significantly promoting the differentiation of NSCs into neurons. In vivo studies showed that the implanted NSCs survived for up to 3 months under the protection of 3D-printed scaffold, differentiated into neurons, formed nerve fibers, and achieved axon regeneration, thus significantly improving the motor function of hind limbs in SCI rats. In addition to simulating the parallel arrangement structure of nerve tracts in the spinal cord, the researchers also developed several conductive bio-inks to mimic the electrophysiological function of the spinal cord to meet the requirements of nerve electrical signal transmission [265, 266]. The innovative 3D-bioprinted conductive spinal cord-like scaffold is similar in conductivity and mechanical characteristics to the spinal cord's native tissue. The conductive bionic scaffold significantly aided the recovery of motor function after SCI in the rat model of complete spinal cord transection injury by inhibiting astrocytes, promoting neuron differentiation at the injured site, and forming rich nerve fibers and myelin sheaths [266]. Researchers also created a supramolecular (SM) bio-ink and printed a scaffold resembling the spinal cord with NSCs and OSMI-4, a small molecule O-GlcNAc transfer inhibitor, to imitate successful neuronal differentiation of nerve cells under a complicated pathological environment (figures 19(g)–(m)) [267]. By providing the dynamic matrix and molecular release source of OSMI-4, the scaffold significantly improved the interaction of NSCs and enhanced their differentiation into neurons. All these works emphasized that 3D bioprinting manufacturing, which can load nerve cells and biological agents to imitate the structure and function of spinal cord tissue, is a promising treatment strategy for SCI repair.
Figure 19. 3D-bioprinted NSC-laden scaffolds and their applications in SCI repair. (a)–(f) Schematic illustration of the NSC-laden bioprinted spinal cord-like construct, and in vivo studies for SCI repair after 12 weeks of implantation. Immunostaining with GFP (green) and Tuj1 (red) in (d), GFP (green) and NF (red) in (e), as well as GFP (green) and Oligo2 (red) in (f) displayed the survival and neuronal differentiation, mature neuron formation, as well as oligodendroglia differentiation of the implanted NSCs in the lesion area, respectively. Reprinted from [26], Copyright (2021), with permission from Elsevier. (g)–(m) Schematic representation of 3D-printed SM hydrogel scaffolds loaded with NSCs and OSMI-4 (SM-OSMI-4 + NSCs), and in vitro and in vivo studies for SCI repair. Live/Dead staining in (j) showed survival of NSCs in the 3D-bioprinted scaffold after 7 d of culture. Immunofluorescence staining with GFAP (green) and Tuj1 (red) in (k), NF (red) in (l), and GAP43 (red) in (m) clarified the neurofilament regeneration, and the axon regeneration in the lesion area of the SM-OSMI-4 + NSCs group after two months of implantation. DAPI (blue) labeled the nucleus. Scale bars in (k)–(m): 1 mm. Reprinted from [267], Copyright (2022), with permission from Elsevier.
Download figure:
Standard image High-resolution image6. Challenges and perspectives
In this review, we discussed how 3D printing technology can be applied to tissue engineered nerve repair of peripheral nerve and SCI. It is possible to imitate the mechanical, structural, and biochemical characteristics of natural tissue using this reliable AM method. Compared with other technologies, 3D printing technology has the following unique advantages in fabricating nerve scaffolds: (1) high controllability: Scaffolds with different shapes and structures can be designed according to requirements, and parameters such as pore size and distribution can be controlled, making it possible to customize scaffolds that meet individual needs, (2) high production efficiency: 3D printing technology can significantly shorten the fabrication time, improve production efficiency, and reduce manufacturing costs compared with traditional fabrication methods, (3) good repeatability: 3D printing technology can ensure that the prepared scaffold has a consistent shape and structure, which can greatly improve the repeatability and consistency of scaffold fabrication, and (4) ability to create complex structures: 3D printing technology can produce very complex structures, such as hollow tubes, branching structures, etc, which can simulate the natural structures and morphology of nerve tissue and improve the effectiveness of nerve regeneration. Complex nerve scaffolds could be created thanks to the rapid development and ongoing innovation of 3D printing, which has broken the limitations of conventional technologies in the fabrication of advanced neural tissue engineering scaffolds. However, several challenges continue to be difficult and are anticipated to be overcome in the future: (1) natural peripheral nerves have a multi-scale hierarchical structure, and 3D printing scaffolds are expected to accurately mimic the structure of natural peripheral nerve tissue. Nevertheless, the majority of extrusion-based 3D printing scaffolds can only simulate the hierarchical structure at low levels and have a limited print resolution. Thus, more sophisticated micro-extrusion nozzles should be created to enable the fabrication of nerve scaffolds with higher resolution, and even to achieve multi-scale 3D printing; (2) the cell bodies and axons of neurons make up the gray matter and white matter of the natural spinal cord, respectively, presenting heterogeneous structures with multiple functions. However, integrated spinal cord scaffolds with various functions are difficult to produce. Therefore, better 3D printing strategies should be adopted to create customized scaffolds with complex features to achieve multi-component 3D printing; (3) in long-distance PNI, due to the lack of sufficient biochemical signal guidance, it is difficult for the injured nerve to regenerate across the chasm, even with the introduction of NGCs. Thus, superior 3D printing technology should be invented to 'shorten' the distance of nerve damage by incorporating the required biochemical cues in situ during the printing process; (4) efficient electrical cue conduction is crucial for nerve regeneration, but the conductive/piezoelectric materials used in current 3D-printed scaffolds are non-degradable. Therefore, it is necessary to develop novel, biocompatible, and printable materials with specific functions; (5) it is crucial to provide a scaffold with adequate vascularization so that enough oxygen and nutrients can be transported during nerve regeneration, but few scaffolds are specially prepared to achieve nerve regeneration with the necessary vascularization. Hence, to produce a scaffold with increased nerve regeneration and enhanced angiogenesis, appropriate strategies are required, such as the controlled release of angiogenesis factor and the development of vascular-like channels in the scaffold. When we look forward to the future of 3D printing constructs for the repair of peripheral nerve and spinal cord injuries, there are several topics worth looking forward to (figure 20).
Figure 20. The future development of 3D printing nerve constructs.
Download figure:
Standard image High-resolution image6.1. Autologous nerve fragment assists the application of NGC in long-distance peripheral nerve repair
Although tissue engineered NGCs can partly simulate the structure and function of natural nerves in long-distance peripheral nerve repair, their therapeutic effect is still not satisfactory. Regarding the management of nerve injury, there are two facts. One is that a biological network with a variety of elements makes up the perfect microenvironment for nerve regeneration, and that, to date, the autologous nerve is the most efficient means to create such a microenvironment. The other is that although the source of autografts with appropriate size and length is limited, small autologous nerves or nerve fragments are relatively easy to obtain in clinical practice. Therefore, it may be a very promising way to treat long-distance PNI by segmentally embellishing autologous nerve fragments into the existing NGC to provide a natural microenvironment. It has been proposed that the small autologous nerve itself is also a kind of damaged nerve, which can lead to the proliferation of SCs and the secretion of neurotrophic factors [268]. In order to repair sciatic nerve defects in rats, Wang et al developed a novel nerve graft that included a chitin conduit and small autologous nerves [269]. This nerve graft significantly encouraged sciatic nerve and myelin sheath regeneration, decreased target muscle atrophy, and successfully restored nerve function. Based on the above ideas, we can take advantage of 3D printing technology to incorporate the autologous nerve fragments into the NGCs on demand and at equal intervals during the manufacturing process, thus simplifying the long-distance peripheral nerve repair to the short-distance PNI repair.
6.2. Self-powered 3D printing scaffold provides effective electrical cues
The electrical cue is a very critical factor in nerve repair and regeneration. It has been shown that SCs grown on PPy/SF composite scaffolds exhibit enhanced vitality, proliferation, and migration when subjected to external ES, as well as up-regulation of neurotrophic factors [270]. In addition, conductive PPy/SF NGC and ES can synergistically promote axon regeneration and myelination in vivo. However, in clinical practice, how to easily introduce ES to the injured site without causing infection and inflammation is still a great challenge. By combining 3D printing and electrospinning technology, Chen et al created a biodegradable high-performance 3D piezoelectric scaffold with wireless ES capability that is driven by ultrasonic waves [271]. It can function as a controlled electrical stimulator as well as a biodegradable scaffold for tissue engineering. The programmable US stimulus can provide controllable ES in vivo, and the duration and intensity can be adjusted. This high-performance piezoelectric scaffold has been successfully applied in the repair of SCI in rats, which provides a new idea for developing a self-powered and biodegradable neural tissue engineering scaffold to achieve non-invasive ES.
6.3. Proper vascularization guarantees the real nerve repair
As we all know, rich vascular networks are distributed throughout neural tissues to promote the transportation of nutrients and the removal of metabolic wastes, which is crucial for maintaining long-term neural cell function. Nerve injury not only destroys the neural circuit, but also breaks the integrity of the microvascular system. In vivo, NGCs need vascular invasion to maintain the basic survival of a large number of SCs to encourage axon regeneration of peripheral nerves [272]. Hobson incorporated vascular endothelial growth factor (VEGF)-loaded MA into a silicone tube and promoted the regeneration of myelinated axons during the repair of sciatic nerve defects [273]. Liu et al constructed a photosensitive hydrogel scaffold laden with stromal cell-derived factor-1α and Taxol, which can induce endothelial cell migration and promote the neurite extension of neurons in vitro [274]. The vascular repair and descent, as well as the axon regeneration of the spinal cord in the lesion area, were all synergistically stimulated when the scaffold was applied to the SCI model, which ultimately improved the motor function of the rats' hind limbs. Lee et al used inkjet printing to create double-layered 3D collagen scaffolds with NSCs and VEGF-releasing fibrin gel, and realized the time-released delivery of VEGF, leading to the promoted proliferation and migration of NSCs in scaffolds, and inducing the morphological changes of NSCs that finally displayed an elongated shape with a neurite-like extension [275]. 3D bioprinting has a high degree of spatial deposition controllability, and it can manipulate multiple types of materials/cells/biomolecules to deposit on demand in the same time and space, such as co-printing of nerve cells and vascular endothelial cells, and/or co-loading of neurotrophic factors and VEGF, leading to creating nerve constructs with complex geometry and diverse functions for the real neural repair.
6.4. In situ 3D printing technology accelerates clinical transformation
Although 3D printing technology has made some research progress in the repair of the spinal cord and peripheral nerve injuries, there is still much to be done in terms of practical application. The advancement of 3D printing technology has facilitated the creation of sophisticated imaging techniques for spotting damaged nerves, intricate algorithms for reconstructing transected nerves, and extremely complex machinery that can in situ print nerve tissue using various bio-ink combinations [276]. Kaplan et al combined 3D printing technology with microcomputed tomography scanning to create PLGA/PLA scaffolds with highly ordered and anatomically personalized architecture, which was specifically customized for SCI [277]. Studies conducted in vitro and in vivo demonstrated that these orientated scaffolds could direct axons to linear conformations and facilitate the development of iPSC-derived neurons. Considering the complexity of neural tissue, in situ 3D printing that can construct patient-specific structures with high fidelity and accurately locate biological factors is the future development direction, and also conforms to the development of personalized medicine.
7. Summary
The application of tissue engineered nerve grafts in peripheral nerve and spinal cord regeneration has evolved greatly with the advent of 3D printing technology and novel materials. The most common ways to print nerve grafts and their uses in treating nerve damage are sketched in this review. Additionally, it is anticipated that this work will make it simpler for readers to choose the optimal scaffold materials, bio-inks, and 3D printing procedures, leading to bionics with a structure and function that are more similar to genuine nerves. To enhance the functionality of printing scaffolds, cutting-edge technologies, optimal material systems, and the incorporation of cells and biological elements are essential. This will facilitate the evolution of 3D-printed nerve constructs and accelerate the clinical transformation of nerve injury treatment.
Acknowledgments
This work was financially sponsored by the National Key Research and Development Program of China (2018YFA0703000), the National Natural Science Foundation of China (No. U1909218), the Joint Funds of Guangdong Basic and Applied Basic Research Foundation (2019A1515110261), the Special Projects in Key Fields from the Department of Education of Guangdong Province (2022ZDZX2059), the Dongguan Science and Technology of Social Development Program (20221800905072).