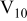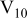Abstract
The inhibition of Al alloy corrosion by vanadates was studied in this work. Vanadium speciation is very complicated and vital to the inhibition efficacy. Critical conditions for decavanadate polymerization from clear metavanadate solutions were investigated. Decavanadate only formed when metavanadate was added to solutions of pH 3 or less. It was not possible to change the pH of a metavanadate solution without forming decavanadates, creating an orange-colored solution. According to  nuclear magnetic resonance, monovanadates were present only in clear metavanadate solutions; orange solutions always contained decavanadates and never contained monovanadates. Orange decavanadate solutions containing
nuclear magnetic resonance, monovanadates were present only in clear metavanadate solutions; orange solutions always contained decavanadates and never contained monovanadates. Orange decavanadate solutions containing  at pH 8.71 exhibited no significant inhibition of the oxygen reduction reaction and increasing decavanadate concentration was detrimental. In contrast, clear metavanadate solutions containing monovanadate exhibited strong inhibition of the oxygen reduction reaction, to a level similar to chromate. At a fixed pH, increased
at pH 8.71 exhibited no significant inhibition of the oxygen reduction reaction and increasing decavanadate concentration was detrimental. In contrast, clear metavanadate solutions containing monovanadate exhibited strong inhibition of the oxygen reduction reaction, to a level similar to chromate. At a fixed pH, increased  concentration in clear metavanadate solutions increased inhibition efficiency.
concentration in clear metavanadate solutions increased inhibition efficiency.
Export citation and abstract BibTeX RIS
High-strength aluminum alloys are used widely in structural aircraft applications because of the combination of good mechanical properties and light weight.1, 2 However, aluminum alloys are prone to localized corrosion if exposed to aggressive environments containing chloride ions.3–6 Current protection schemes are based on the chemistry of chromate oxoanions. Chromates and dichromates in aqueous solutions, as conversion coatings or as pigment in primers, impart excellent corrosion protection to most aluminum alloys.7–14 The mechanism thought to be responsible for such potent inhibition involves the formation of a  monolayer at the surface which impedes both oxygen and further
monolayer at the surface which impedes both oxygen and further  electrochemical reduction.14–20 After the formation of this
electrochemical reduction.14–20 After the formation of this  monolayer, a surface film with a mixed
monolayer, a surface film with a mixed  oxidation state develops.18 The mixed oxidation state is responsible for the reported self-healing capabilities of chromate-based systems.7, 9, 13, 18, 21–24 Labile
oxidation state develops.18 The mixed oxidation state is responsible for the reported self-healing capabilities of chromate-based systems.7, 9, 13, 18, 21–24 Labile  can be released from the coating and migrate to a damaged region where electrochemical reduction to
can be released from the coating and migrate to a damaged region where electrochemical reduction to  can occur, protecting the surface from further corrosion.7, 8, 12, 18, 21, 23, 24 Despite their excellent performance, several regulations prohibit the use of
can occur, protecting the surface from further corrosion.7, 8, 12, 18, 21, 23, 24 Despite their excellent performance, several regulations prohibit the use of  technologies due to their high toxicity and carcinogenic effects.25 The pressure imposed by these regulations has been a significant driving force promoting the development of chromate-free alternatives. As summarized by Kendig and Buchheit, compounds derived from almost 40 elements of the periodic table and combinations of them have been investigated.9, 14 However, no suitable alternatives for high-strength Al alloys have been reported to date.
technologies due to their high toxicity and carcinogenic effects.25 The pressure imposed by these regulations has been a significant driving force promoting the development of chromate-free alternatives. As summarized by Kendig and Buchheit, compounds derived from almost 40 elements of the periodic table and combinations of them have been investigated.9, 14 However, no suitable alternatives for high-strength Al alloys have been reported to date.
Vanadium-based oxyanions, also referred to as vanadates, have been investigated as corrosion inhibitors for Al alloys. However, they have not gained much attention probably due to the relatively large solubility of vanadium oxides in aqueous solutions.9, 14, 26, 27 Smith et al. explored the release kinetics and protection performance of vanadate-based pigments in epoxy-coated AA2014-T6 panels. The authors concluded that vanadates are effective pigments for protecting Al alloys.28 A later investigation by Cook et al. screened and compared several candidate inhibitors including vanadates, molybdates, and ions of rare earth elements like Ce, Y, and La.29 The authors concluded that in aqueous solutions at neutral to basic pHs, vanadates gave the best performance, reaching, in some cases, chromate-like levels. Interestingly, at lower pHs no inhibitor imparted good protection; however, vanadates were among the best performing compounds. Buchheit et al. developed a surface conversion process for aluminum alloys based on acidic vanadium formulations, which imparted protection to AA2024-T3 coated panels to some extent.26 In addition, the authors analyzed the behavior of aqueous solutions containing vanadium oxoanions and concluded that metavanadates, i.e., vanadate oligomers including  ,
,  ,
,  , and
, and  coordination, are not potent inhibitors of the oxygen reduction reaction (ORR) but significantly lower the anodic dissolution kinetics. Buchheit et al. also explored the use of vanadates as a counterion in hydrotalcite coatings. The authors reported that migration of vanadates from the coating can impart active corrosion protection.30 Recently, Chambers et al. studied the synergism of several binary and ternary mixtures including vanadates, phosphates, molybdates, and several rare earths. The authors concluded that the maximum synergistic effect occurred for vanadate-phosphate solutions in a
coordination, are not potent inhibitors of the oxygen reduction reaction (ORR) but significantly lower the anodic dissolution kinetics. Buchheit et al. also explored the use of vanadates as a counterion in hydrotalcite coatings. The authors reported that migration of vanadates from the coating can impart active corrosion protection.30 Recently, Chambers et al. studied the synergism of several binary and ternary mixtures including vanadates, phosphates, molybdates, and several rare earths. The authors concluded that the maximum synergistic effect occurred for vanadate-phosphate solutions in a  ratio.31 However, all of these studies of inhibition by vanadates lacked information regarding vanadate speciation in the test solutions, and interpretations were based on the analysis of speciation diagrams,which do not consider metastable phases.
ratio.31 However, all of these studies of inhibition by vanadates lacked information regarding vanadate speciation in the test solutions, and interpretations were based on the analysis of speciation diagrams,which do not consider metastable phases.
The coordination chemistry of vanadium oxoanions in aqueous solutions is rather complex. It involves several protonation/deprotonation reactions, as well as polymerization to form oligomers of varied molecular weight, depending upon pH and concentration.32–36 In general, all the polymerized species are referred to as isopolyanions or isopolymetallates.34, 36–38
In part, as a consequence of the role played by vanadates in modifying several biochemical processes, vanadate speciation has been the subject of several review studies that characterized the system in detail.39–47 Initially, techniques such as ultraviolet/visible spectroscopy, pH measurements, and electrochemical studies were employed to determine the stoichiometry of the equilibria of the different vanadate species.48, 49 However, the information extracted from those techniques is rather limited and possesses virtually no direct evidence of the coordination structure of the different isopolyanions and in some cases, was proven to be incorrect.41, 42 It was not until the maturing of nuclear magnetic resonance (NMR) spectroscopy that the exact structure and concentration of the different V oligomers present in aqueous solutions were understood.42 Nevertheless, there is still some debate regarding existence and coordination of some species such as  .42–44
.42–44
Modern high-field NMR gives high-quality structural data and quantitative information regarding speciation and concentration of a large variety of compounds in aqueous and nonaqueous solvents.50 The only limitation of NMR is the inability of detecting minor components with overlapping or coincident chemical shifts.50 According to  and
and  NMR spectroscopy, the observed oligomers in the range of pH 7–9 and at varied vanadate concentrations are the monovanadates
NMR spectroscopy, the observed oligomers in the range of pH 7–9 and at varied vanadate concentrations are the monovanadates  , divanadates
, divanadates  , cyclic tetravanadates
, cyclic tetravanadates  , and cyclic pentavanadates
, and cyclic pentavanadates  .42–44 In contrast, decavanadates are stable in the pH range from 2 to 6 but can form in solutions of higher pH due to local acidification of the solution.32, 36, 39, 41, 44 Polymerization to
.42–44 In contrast, decavanadates are stable in the pH range from 2 to 6 but can form in solutions of higher pH due to local acidification of the solution.32, 36, 39, 41, 44 Polymerization to  is rapid and the mechanism of formation is not well understood. Depolymerization of
is rapid and the mechanism of formation is not well understood. Depolymerization of  , on the other hand, is very slow.39 Heating can be used to precipitate decavanadate crystals out of aqueous solutions.51
, on the other hand, is very slow.39 Heating can be used to precipitate decavanadate crystals out of aqueous solutions.51
The results of prior studies on corrosion inhibition by vanadates are clouded by a lack of understanding of speciation, the complexity of the hydrolysis of vanadates in aqueous solutions, and the existence of metastable equilibria. The main objective of this work is to understand which vanadate oligomer imparts the best corrosion protection for AA2024-T3. Vanadate speciation as a function of pH and concentration is investigated by  NMR. The influence of decavanadate formation, solution aging, and bulk pH on corrosion inhibition of AA2024-T3 is also addressed.
NMR. The influence of decavanadate formation, solution aging, and bulk pH on corrosion inhibition of AA2024-T3 is also addressed.
Experimental
Materials and sample preparation
Commercially available sodium vanadium oxide, 98%  , and reagent-grade sodium chloride from Sigma-Aldrich were used. All solutions were prepared with
, and reagent-grade sodium chloride from Sigma-Aldrich were used. All solutions were prepared with  deionized water.
deionized water.
To reduce the possibility of composition or heat-treatment artifacts, samples were cut from two different AA2024-T3 panels (nominal composition  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  , balance Al) that were 1 and
, balance Al) that were 1 and  thick, respectively. The samples were mounted in epoxy resin; ground through 1200 grit
thick, respectively. The samples were mounted in epoxy resin; ground through 1200 grit  papers (Buehler), and polished with 3 and
papers (Buehler), and polished with 3 and  diamond paste (Buehler). Ethyl alcohol (
diamond paste (Buehler). Ethyl alcohol ( water) was used as a lubricant during all the surface preparation stages to minimize corrosion damage during grinding and polishing.
water) was used as a lubricant during all the surface preparation stages to minimize corrosion damage during grinding and polishing.
Determination of the minimum pH that triggers orange  polymerization
polymerization
As-prepared,  solutions remain colorless with a pH that linearly increases with total vanadium concentration (Fig. 1). Acidification with concentrated
solutions remain colorless with a pH that linearly increases with total vanadium concentration (Fig. 1). Acidification with concentrated  to adjust bulk pH produces a change in color to orange indicating the formation of decavanadates. The determination of the minimum pH that causes
to adjust bulk pH produces a change in color to orange indicating the formation of decavanadates. The determination of the minimum pH that causes  formation was investigated. At the same time it was possible to determine whether the bulk pH can be adjusted without introducing color change. Aliquots of colorless
formation was investigated. At the same time it was possible to determine whether the bulk pH can be adjusted without introducing color change. Aliquots of colorless  solutions were added to beakers containing exclusively
solutions were added to beakers containing exclusively  solutions with initial pH
solutions with initial pH  varying from 1 to 12. The
varying from 1 to 12. The  solutions were not buffered to avoid any possible complexation between vanadium and the buffer media. Therefore,
solutions were not buffered to avoid any possible complexation between vanadium and the buffer media. Therefore,  was adjusted and measured immediately before mixing. Final
was adjusted and measured immediately before mixing. Final  concentrations of 100, 50, or
concentrations of 100, 50, or  were obtained by varying the injected volume. The final pH after mixing,
were obtained by varying the injected volume. The final pH after mixing,  , was monitored and determined as a function of
, was monitored and determined as a function of  .
.
Figure 1. pH of clear, as-dissolved solutions as a function of  concentration.
concentration.
NMR spectroscopy
Procedures for  NMR described in the literature were followed.39, 42–47 High-resolution
NMR described in the literature were followed.39, 42–47 High-resolution 
 spectra were obtained at room temperature utilizing a Bruker DPX
spectra were obtained at room temperature utilizing a Bruker DPX  superconducting magnet. A broadband direct detection
superconducting magnet. A broadband direct detection  probe with a corresponding
probe with a corresponding 
 pulse duration was used. The
pulse duration was used. The  chemical shifts were referenced to an external standard 20% v/v
chemical shifts were referenced to an external standard 20% v/v  in
in 
 . All spectra were acquired with an accumulation of 1024 transients using a spectral window of
. All spectra were acquired with an accumulation of 1024 transients using a spectral window of  , an acquisition time of
, an acquisition time of  , and a relaxation delay of
, and a relaxation delay of  . The following processing parameters were applied to each spectrum prior to integration:
. The following processing parameters were applied to each spectrum prior to integration:  line broadening, zero filling, and baseline corrections.
line broadening, zero filling, and baseline corrections.
Speciation of clear metavanadate solutions and orange solutions containing decavanadate at varied concentrations was compared at the same high pH. Decavanadate solutions were prepared by acidifying clear metavanadate solutions to pH 4 to obtain an orange solution and readjusting the electrolyte to pH 8.71 using  . The resulting solution remained colored at the high pH. Metavanadate solutions were also directly adjusted to higher pHs, and these solutions remained clear. The effects of pH on speciation and corrosion performance of clear metavanadate solutions were also analyzed by increasing the pH of
. The resulting solution remained colored at the high pH. Metavanadate solutions were also directly adjusted to higher pHs, and these solutions remained clear. The effects of pH on speciation and corrosion performance of clear metavanadate solutions were also analyzed by increasing the pH of  solutions with pH 7.8–10.
solutions with pH 7.8–10.
Electrochemical tests
All electrochemical tests were carried out using either a Gamry PC3/300 or a VoltaLab PGP-201 potentiostat in a three-electrode array. A platinum mesh counter electrode and a standard calomel reference electrode (SCE) were used in all experiments. A Luggin capillary filled with an agar-agar gel made with  was used in all tests. Samples were cleaned and degreased with ethyl alcohol before testing. Each experiment was repeated at least in triplicate. Cathodic polarization experiments were carried out in solutions that were bubbled with air for
was used in all tests. Samples were cleaned and degreased with ethyl alcohol before testing. Each experiment was repeated at least in triplicate. Cathodic polarization experiments were carried out in solutions that were bubbled with air for  prior to the experiment and stirred during the experiment to generate a more reproducible limiting current for the ORR. The potential sweep started
prior to the experiment and stirred during the experiment to generate a more reproducible limiting current for the ORR. The potential sweep started  above the open circuit potential (OCP) and was stopped when the current density reached
above the open circuit potential (OCP) and was stopped when the current density reached  . A scan rate of
. A scan rate of  was used and the total exposed area was approximately
was used and the total exposed area was approximately  . Corrosion rate at OCP was assessed from the polarization resistance
. Corrosion rate at OCP was assessed from the polarization resistance  determined using the linear polarization technique (LPT). The potential was scanned at
determined using the linear polarization technique (LPT). The potential was scanned at  over a range
over a range  relative to the OCP. LPT measurements were repeated each
relative to the OCP. LPT measurements were repeated each  for
for  .
.
Long-term OCP exposure
Samples were immersed in chloride solution containing decavanadate or metavanadate solution for 14 days. The solutions were renewed every  to reduce the effects of changes in pH or speciation.
to reduce the effects of changes in pH or speciation.
Results and Discussion
Determination of the minimum pH that triggers  forma tion
forma tion
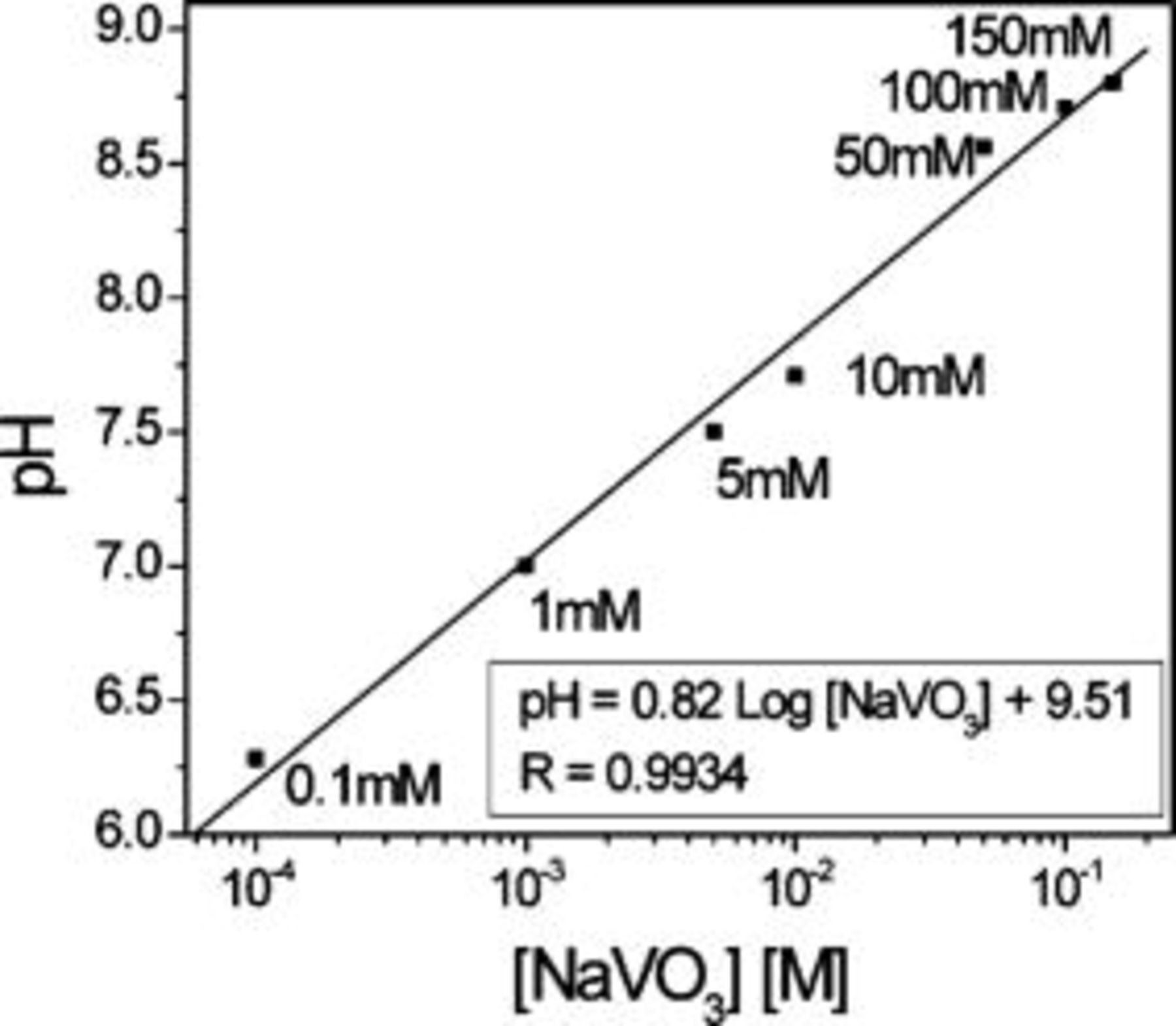
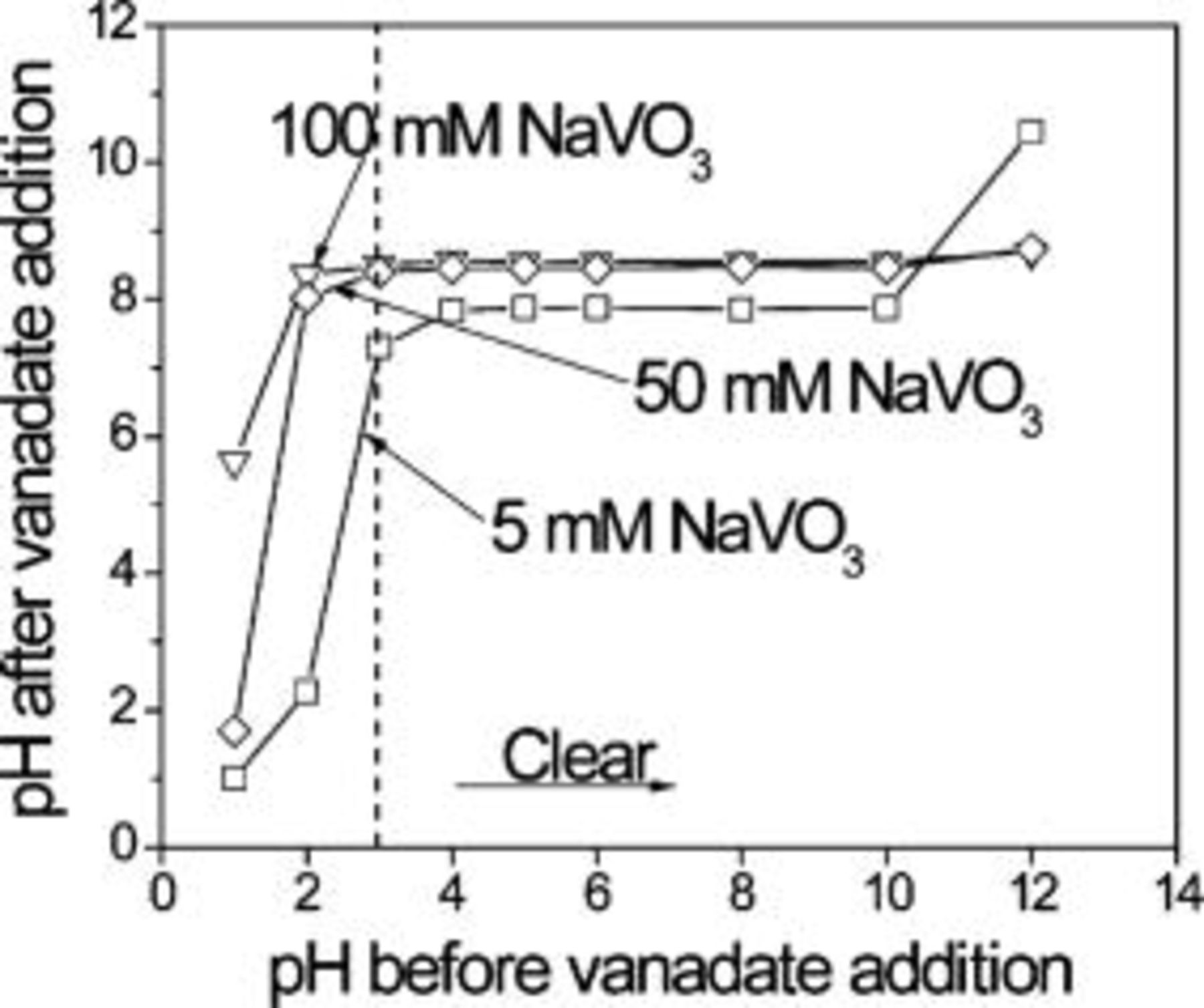
The objective of these experiments was to determine the critical conditions (i.e., initial pH and final vanadate concentration) that cause decavanadate formation, as evidenced by a change in color from clear to orange. The injection of the concentrated clear  solution was made slowly and recorded with a digital camera. Figure 2 shows the
solution was made slowly and recorded with a digital camera. Figure 2 shows the  as a function of
as a function of  . The color change associated with
. The color change associated with  formation was only triggered for
formation was only triggered for  , independent of final vanadate concentration. Interestingly, for
, independent of final vanadate concentration. Interestingly, for  and
and  final vanadate concentrations,
final vanadate concentrations,  was equal to 8.4 independent of
was equal to 8.4 independent of  for
for  . For
. For  total vanadate,
total vanadate,  was equal to 7.6 independent of
was equal to 7.6 independent of  for
for  . In other words, to create vanadate solutions of concentration
. In other words, to create vanadate solutions of concentration  or higher with pH less than 7.5, the vanadates must be mixed with solutions having pH less than 3–4. Since this pH range is above the pH for color change, a direct implication of these findings is the practical impossibility of adjusting the pH of clear
or higher with pH less than 7.5, the vanadates must be mixed with solutions having pH less than 3–4. Since this pH range is above the pH for color change, a direct implication of these findings is the practical impossibility of adjusting the pH of clear  solutions, without producing a change in color and speciation. Therefore, previous studies that investigated inhibition by vanadates at pH 7 actually used solutions containing decavanadate.26, 29, 30 Exact speciation of these solutions are addressed below. Once formed, however, orange decavanadate solutions can be adjusted to high pH without completely depolymerizing the
solutions, without producing a change in color and speciation. Therefore, previous studies that investigated inhibition by vanadates at pH 7 actually used solutions containing decavanadate.26, 29, 30 Exact speciation of these solutions are addressed below. Once formed, however, orange decavanadate solutions can be adjusted to high pH without completely depolymerizing the  formed during acidification. Therefore, it was possible to analyze and compare clear metavanadate and orange decavanadate solutions at a common pH only at high pH. Here solutions were adjusted to pH 8.71, which corresponds to the as-dissolved pH of
formed during acidification. Therefore, it was possible to analyze and compare clear metavanadate and orange decavanadate solutions at a common pH only at high pH. Here solutions were adjusted to pH 8.71, which corresponds to the as-dissolved pH of  .
.
Figure 2. Final pH of solutions formed by mixing metavanadate with solutions of varying initial pH. The concentration refers to the final vanadate concentration after mixing.
The fact that a local environment with a  triggers formation of decavanadates not only has direct implications in sample preparation procedures, but it might also impact coating schemes based on vanadates. The case of an epoxy coating containing a metavanadate pigment can be used as a simple example. If a scratch through the coating is exposed to an aggressive electrolyte, release of a vanadate species from the coating to the damaged area will occur. If the pH of the electrolyte is lower than 3, released metavanadates will polymerize to form decavanadates. In such a case, decavanadates will have to protect the exposed bare metal from further corrosion. On the other hand, if the pH of the aggressive electrolyte is greater than 4, metavanadates will be the species released and present in solution.
triggers formation of decavanadates not only has direct implications in sample preparation procedures, but it might also impact coating schemes based on vanadates. The case of an epoxy coating containing a metavanadate pigment can be used as a simple example. If a scratch through the coating is exposed to an aggressive electrolyte, release of a vanadate species from the coating to the damaged area will occur. If the pH of the electrolyte is lower than 3, released metavanadates will polymerize to form decavanadates. In such a case, decavanadates will have to protect the exposed bare metal from further corrosion. On the other hand, if the pH of the aggressive electrolyte is greater than 4, metavanadates will be the species released and present in solution.
Nuclear magnetic resonance spectroscopy (NMR)
 NMR was used to determine quantitatively the speciation of vanadate solutions. Decavanadates were prepared by first acidifying clear metavanadate solutions to pH 4 and then readjusting to pH 8.71. Since decavanadate solutions are not thermodynamically stable in these conditions, solutions were prepared immediately before NMR analysis.
NMR was used to determine quantitatively the speciation of vanadate solutions. Decavanadates were prepared by first acidifying clear metavanadate solutions to pH 4 and then readjusting to pH 8.71. Since decavanadate solutions are not thermodynamically stable in these conditions, solutions were prepared immediately before NMR analysis.
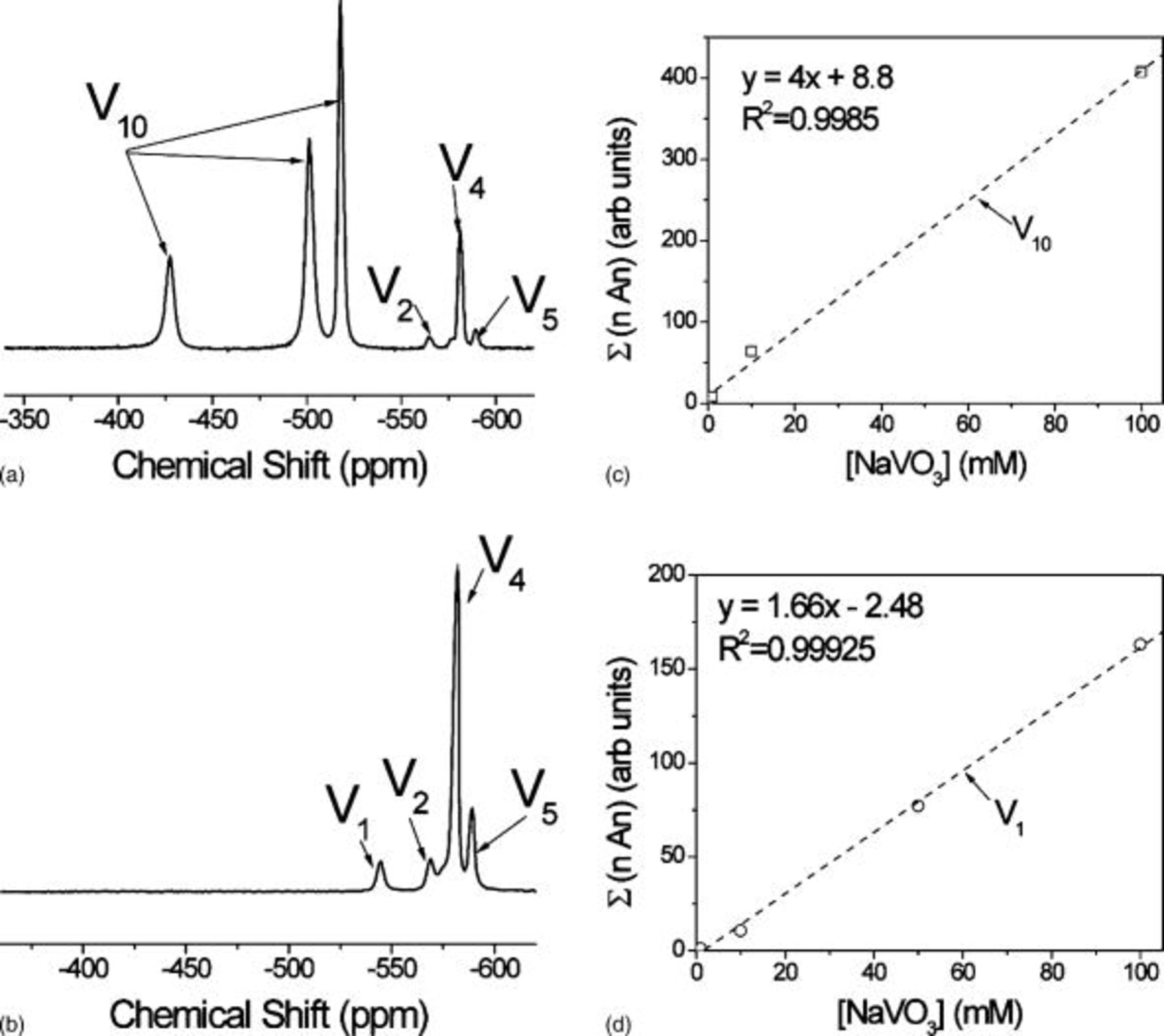
Typical  NMR spectra for a
NMR spectra for a  decavanadate solution and a
decavanadate solution and a  metavanadate solution adjusted to pH 8.71 are shown in Fig. 3a and 3b. The peaks are labeled based on assignments reported in the literature.32–34, 39, 41–47 NMR peaks are proportional to the species concentration.50 Summations of the peak areas of spectra such as those in Fig. 3a and 3b from both decavanadate and metavanadate solutions of varying concentration are given in Fig. 3c and 3d, respectively. In both cases, the sum of
metavanadate solution adjusted to pH 8.71 are shown in Fig. 3a and 3b. The peaks are labeled based on assignments reported in the literature.32–34, 39, 41–47 NMR peaks are proportional to the species concentration.50 Summations of the peak areas of spectra such as those in Fig. 3a and 3b from both decavanadate and metavanadate solutions of varying concentration are given in Fig. 3c and 3d, respectively. In both cases, the sum of  times the area under the peaks, where
times the area under the peaks, where  , 2, 4, 5, or 10, linearly scaled with total vanadium concentration, thereby proving the validity of the peak assignment and providing a calibration of the method.
, 2, 4, 5, or 10, linearly scaled with total vanadium concentration, thereby proving the validity of the peak assignment and providing a calibration of the method.
Figure 3. Assignment of  NMR peaks and calibration: (a) typical
NMR peaks and calibration: (a) typical  NMR spectrum for an orange decavanadate solution, (b) typical
NMR spectrum for an orange decavanadate solution, (b) typical  NMR spectrum for a clear metavanadate solution, (c) calibration curve for orange decavanadate solutions, and (d) calibration curve for clear metavanadate solutions.
NMR spectrum for a clear metavanadate solution, (c) calibration curve for orange decavanadate solutions, and (d) calibration curve for clear metavanadate solutions.
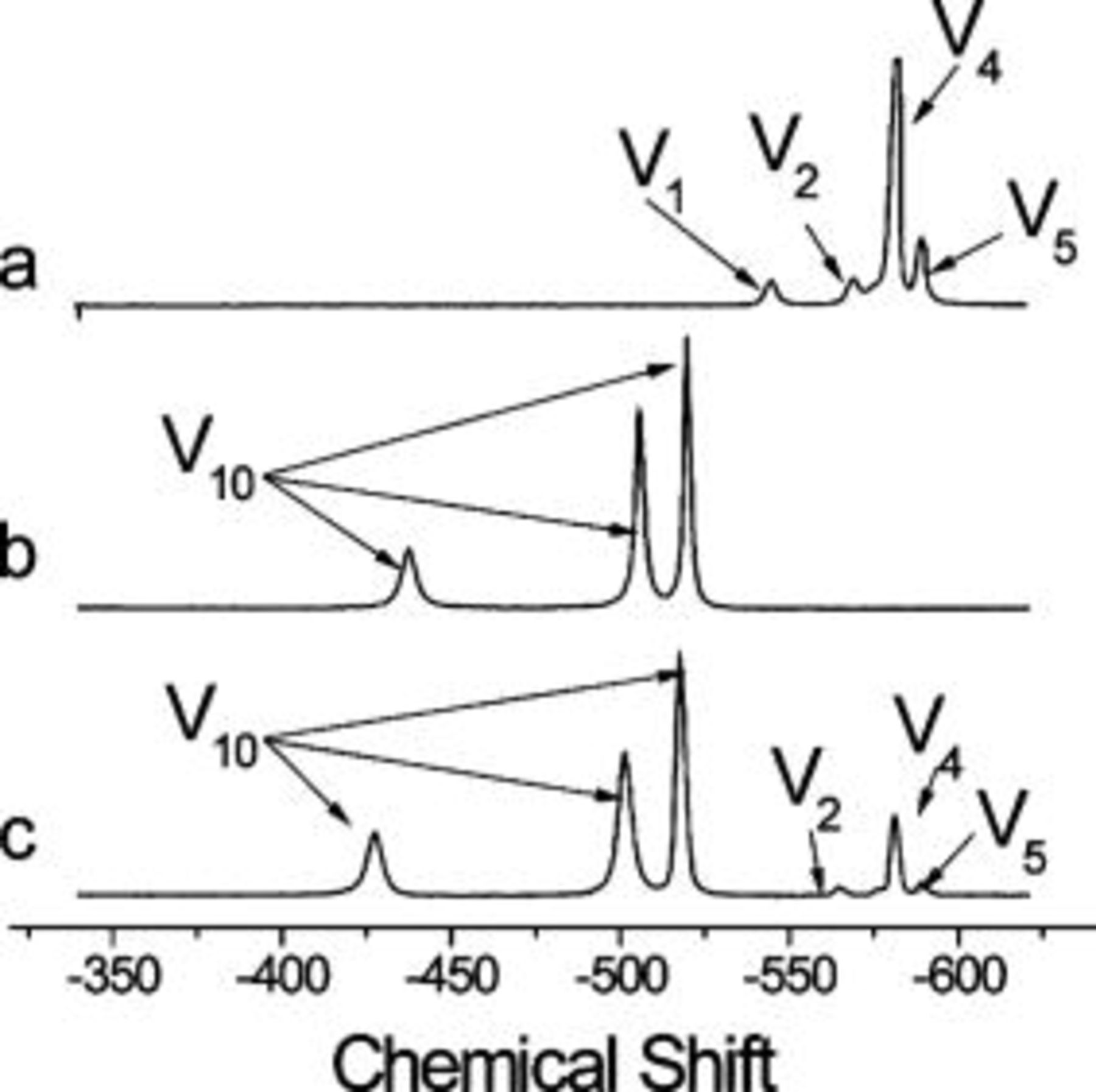
Figure 4 shows changes in speciation associated with pH adjustment. A typical  NMR spectrum of a
NMR spectrum of a  clear metavanadate solution at pH 8.71 is shown in Fig. 4a. The oligomers present in such conditions are the monovanadate
clear metavanadate solution at pH 8.71 is shown in Fig. 4a. The oligomers present in such conditions are the monovanadate  , the divanadate
, the divanadate  , the tetravanadate
, the tetravanadate  , and the pentavanadate
, and the pentavanadate  as has been reported previously.32–34, 41–43, 50 At low concentrations
as has been reported previously.32–34, 41–43, 50 At low concentrations 
 is the predominant species, whereas at higher concentrations, such as the case of Fig. 4a, the oligomers of higher molecular weight become predominant.42, 44 When the clear metavanadate solutions are acidified to pH 4, all the metavanadates polymerize to form an orange decavanadate solution exclusively containing, within the detection limit of the instrument, signal from
is the predominant species, whereas at higher concentrations, such as the case of Fig. 4a, the oligomers of higher molecular weight become predominant.42, 44 When the clear metavanadate solutions are acidified to pH 4, all the metavanadates polymerize to form an orange decavanadate solution exclusively containing, within the detection limit of the instrument, signal from  oligomers (Fig. 4b). Increasing the pH of the decavanadate solutions produces the partial depolymerization of
oligomers (Fig. 4b). Increasing the pH of the decavanadate solutions produces the partial depolymerization of  to give
to give  ,
, , and
, and  (Fig. 4c). At low concentrations
(Fig. 4c). At low concentrations  ,
,  is the predominant species and
is the predominant species and  is the main metavanadate in these solutions with pH adjusted back to high values. As
is the main metavanadate in these solutions with pH adjusted back to high values. As  increases,
increases,  becomes the predominant metavanadate, as in the case of Fig. 4c.
becomes the predominant metavanadate, as in the case of Fig. 4c.
Figure 4.
 NMR spectra of
NMR spectra of  vanadate solution in different conditions: (a) as-dissolved pH 8.71, (b) acidified to pH 4, and (c) acidified and readjusted to pH 8.71.
vanadate solution in different conditions: (a) as-dissolved pH 8.71, (b) acidified to pH 4, and (c) acidified and readjusted to pH 8.71.
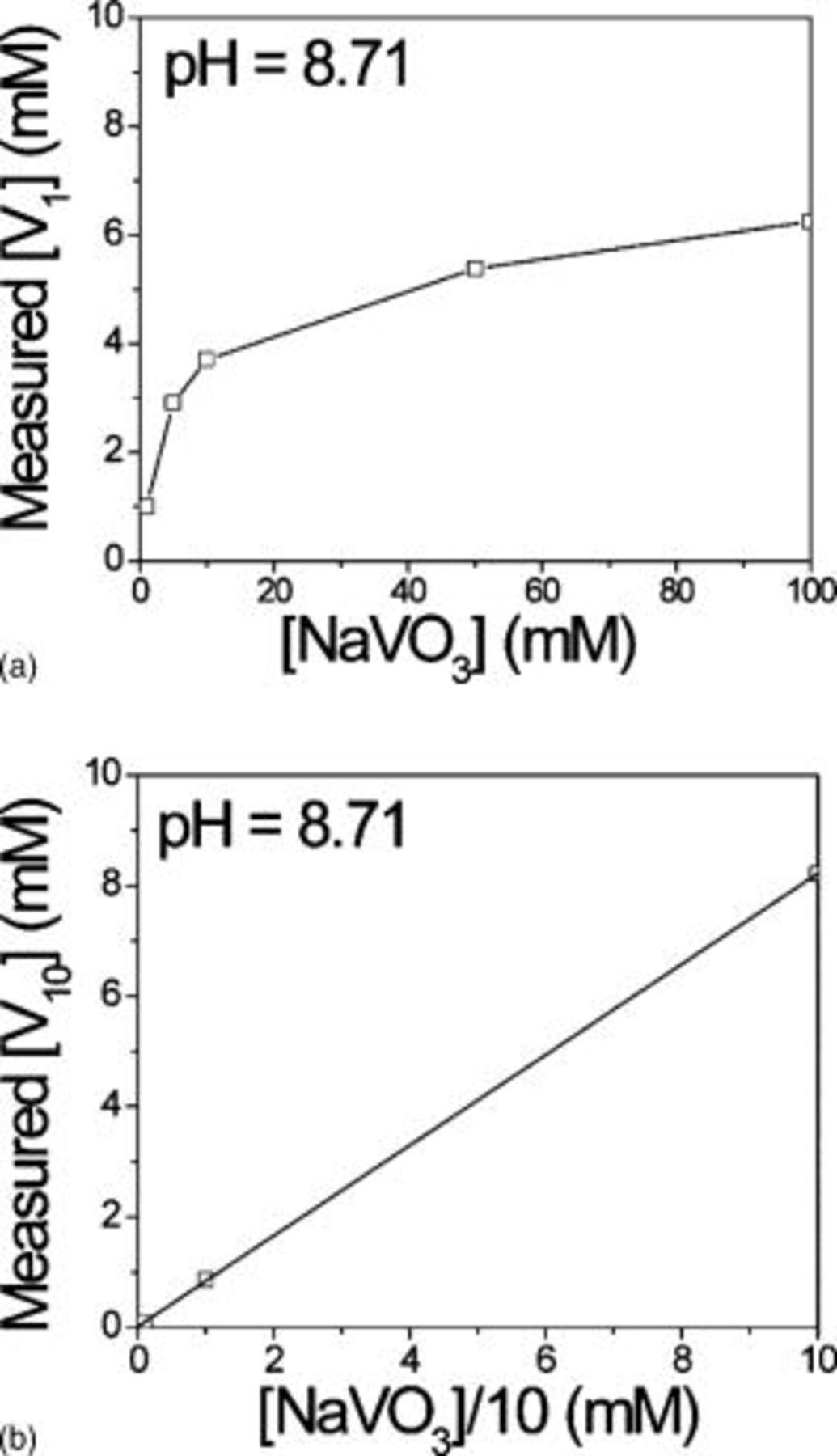
Comparison of Fig. 4a and 4b indicates that clear metavanadate solutions contain vanadate monomers, whereas orange decavanadate solutions at pH 8.71 do not. In all of the orange solutions examined,  was always observed and
was always observed and  was never observed. Figure 5 shows the concentration of
was never observed. Figure 5 shows the concentration of  species in clear metavanadate solutions and the concentration of
species in clear metavanadate solutions and the concentration of  in orange decavanadate solutions as a function of the total vanadate concentration, all at pH 8.71. The concentration of
in orange decavanadate solutions as a function of the total vanadate concentration, all at pH 8.71. The concentration of  linearly scales with total
linearly scales with total  . However, the amount of
. However, the amount of  present in clear metavanadate solutions seems to reach a saturation level of
present in clear metavanadate solutions seems to reach a saturation level of  .
.
Figure 5. Concentration of  and
and  as a function of total
as a function of total  : (a) clear metavanadate solutions and (b) orange decavanadate solutions. All at pH 8.71.
: (a) clear metavanadate solutions and (b) orange decavanadate solutions. All at pH 8.71.
Table I summarizes the chemical shifts, δ, observed for the different oligomers for both decavanadate and metavanadate solutions at pH 8.71. At a fixed pH the chemical shift of the different oligomers does not vary with incremental concentrations of  .32, 42–44 Using chemical shift information, it is possible to determine the state of protonation of the oligomers.43 Within the experimental error of
.32, 42–44 Using chemical shift information, it is possible to determine the state of protonation of the oligomers.43 Within the experimental error of  , the results are in good agreement with Heath et al. and Crans et al.32, 42 Nevertheless, the chemical shifts are about
, the results are in good agreement with Heath et al. and Crans et al.32, 42 Nevertheless, the chemical shifts are about  more negative than commonly tabulated values. The small discrepancies are probably caused by different experimental conditions such as temperature, ionic strength, and/or pH.42, 50 For the clear solutions, the chemical shift of the monovanadates indicates that monoprotonated and diprotonated
more negative than commonly tabulated values. The small discrepancies are probably caused by different experimental conditions such as temperature, ionic strength, and/or pH.42, 50 For the clear solutions, the chemical shift of the monovanadates indicates that monoprotonated and diprotonated  are present. Likewise, for both clear and orange solutions, the chemical shift for the divanadate species indicates that
are present. Likewise, for both clear and orange solutions, the chemical shift for the divanadate species indicates that  and
and  are at equilibrium. As hypothesized by Heath et al. , the slightly more negative chemical shifts for both
are at equilibrium. As hypothesized by Heath et al. , the slightly more negative chemical shifts for both  and
and  may correspond to an equilibrium reaction between cyclic and linear oligomers.42 In addition, for the orange solutions, the position of the three
may correspond to an equilibrium reaction between cyclic and linear oligomers.42 In addition, for the orange solutions, the position of the three  peaks seems to indicate that the decavanadate species at metastable equilibrium are
peaks seems to indicate that the decavanadate species at metastable equilibrium are  and
and  .32
.32
Table I. Chemical shift for the different oligomers present in clear and orange solutions obtained by  NMR.
NMR.
| Species | Chemical shift (ppm) | |
|---|---|---|
| Clear |
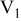
|
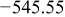
|
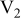
|
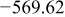
| |
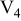
|
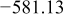
| |
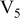
|
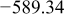
| |
| Orange |
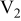
|
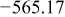
|
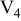
|
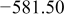
| |
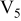
|
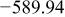
| |
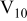
|
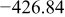
| |
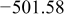
| ||
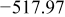
|
Inhibition by metavanadate and decavanadate solutions
The corrosion-inhibiting effects of orange solutions containing decavanadate but no monovanadate and clear solutions containing metavanadates  but no decavanadate were determined at the fixed pH of 8.71. These solutions will be referred to as orange decavanadate and clear metavanadate solutions, respectively, even though the former solutions also contain some metavanadates. Cathodic polarization curves of AA2024-T3 were measured in orange decavanadate and clear metavanadate pH 8.71 solutions containing
but no decavanadate were determined at the fixed pH of 8.71. These solutions will be referred to as orange decavanadate and clear metavanadate solutions, respectively, even though the former solutions also contain some metavanadates. Cathodic polarization curves of AA2024-T3 were measured in orange decavanadate and clear metavanadate pH 8.71 solutions containing  and varying amounts of
and varying amounts of  . Figure 6a compares typical cathodic polarization curves for
. Figure 6a compares typical cathodic polarization curves for  solutions. A cathodic polarization curve in
solutions. A cathodic polarization curve in  with no vanadates is also shown for reference. In the absence of inhibitor, the ORR is not impeded and a limiting current,
with no vanadates is also shown for reference. In the absence of inhibitor, the ORR is not impeded and a limiting current,  , of
, of  is observed. The
is observed. The  obtained in Cu-containing aluminum alloys is significantly larger than in pure Al or in Al alloys not alloyed with Cu.52–54 Although not completely understood, it is generally accepted that the presence of Cu contamination in the passive film on the matrix or on intermetallic particles increases conductivity of the oxide, making the diffusion of oxygen the rate-limiting step.54–56 In pure Al, the alumina layer acts as an electric isolator, causing electron transfer throughout the film to be extremely difficult. The ability to sustain a large cathodic current makes
obtained in Cu-containing aluminum alloys is significantly larger than in pure Al or in Al alloys not alloyed with Cu.52–54 Although not completely understood, it is generally accepted that the presence of Cu contamination in the passive film on the matrix or on intermetallic particles increases conductivity of the oxide, making the diffusion of oxygen the rate-limiting step.54–56 In pure Al, the alumina layer acts as an electric isolator, causing electron transfer throughout the film to be extremely difficult. The ability to sustain a large cathodic current makes  alloys very susceptible to localized corrosion.55 Therefore, if a corrosion inhibitor in the aggressive electrolyte controls the kinetics of oxygen reduction, a diminished corrosion rate is expected.9, 10, 13, 14, 21, 52, 55, 57, 58
alloys very susceptible to localized corrosion.55 Therefore, if a corrosion inhibitor in the aggressive electrolyte controls the kinetics of oxygen reduction, a diminished corrosion rate is expected.9, 10, 13, 14, 21, 52, 55, 57, 58
Figure 6. Inhibition of ORR by vanadate solutions. (a) Cathodic polarization curves of AA2024-T3 in  with no inhibitor, orange decavanadate, or clear metavanadate. (b) Effects of vanadate concentration on inhibition efficiency measured by calculating the cathodic current at
with no inhibitor, orange decavanadate, or clear metavanadate. (b) Effects of vanadate concentration on inhibition efficiency measured by calculating the cathodic current at  SCE.
SCE.
Figure 6a shows that orange decavanadate solutions do not reduce the kinetics of ORR significantly. For a  decavanadate solution, an average
decavanadate solution, an average  of
of  is observed. The effects of
is observed. The effects of  concentration are summarized in Fig. 6b. Comparison of the effects of vanadate on ORR kinetics is complicated by the different shapes of the polarization curves, especially at low potentials. The current density values plotted in Fig. 6b were obtained by extrapolating the linear regions of the cathodic curves to the high potential of
concentration are summarized in Fig. 6b. Comparison of the effects of vanadate on ORR kinetics is complicated by the different shapes of the polarization curves, especially at low potentials. The current density values plotted in Fig. 6b were obtained by extrapolating the linear regions of the cathodic curves to the high potential of  SCE. This is an arbitrary definition and different approaches could have been taken. However, it gives a fair representation of the inhibition efficacy. Higher vanadate concentrations resulted in larger cathodic currents for the orange decavanadate solutions, supporting the concept that decavanadates are poor inhibitors of the ORR. The small amount of inhibition observed in decavanadate solutions is possibly a consequence of the presence of metavanadates such as
SCE. This is an arbitrary definition and different approaches could have been taken. However, it gives a fair representation of the inhibition efficacy. Higher vanadate concentrations resulted in larger cathodic currents for the orange decavanadate solutions, supporting the concept that decavanadates are poor inhibitors of the ORR. The small amount of inhibition observed in decavanadate solutions is possibly a consequence of the presence of metavanadates such as  . As the
. As the  concentration increases, the amounts of
concentration increases, the amounts of  and
and  increase accordingly, lowering the
increase accordingly, lowering the  ratio. It is hypothesized that adsorption of
ratio. It is hypothesized that adsorption of  is somehow impeded by the presence of
is somehow impeded by the presence of  and
and , which lowers the inhibition extent by allowing oxygen to adsorb freely on the local cathodes and then reduce.
, which lowers the inhibition extent by allowing oxygen to adsorb freely on the local cathodes and then reduce.
In contrast to the orange decavanadate solutions, a significant decrease in the rate of oxygen reduction was observed in clear metavanadate solutions. The kinetics of oxygen reduction were reduced by almost 4 orders of magnitude and no diffusion-limiting region was observed (Fig. 6a). In addition, the threshold for hydrogen evolution was shifted toward more negative overpotentials. In further contrast to the orange decavanadate solutions, inhibition performance increased with increasing vanadate concentration (Fig. 6b). Since the orange decavanadate solutions contain no  and the clear metavanadate solutions contain no
and the clear metavanadate solutions contain no  , these results suggest that decavanadates are detrimental or ineffective inhibitors and monovanadates provide the best inhibition. In this regard, the extent of inhibition imparted by monovanadates is similar to that reported for chromates and dichromates in aqueous solutions.21, 23, 57 These data provide no insight on whether the monomers are adsorbed or reduced at the surface. The mechanisms of inhibition by
, these results suggest that decavanadates are detrimental or ineffective inhibitors and monovanadates provide the best inhibition. In this regard, the extent of inhibition imparted by monovanadates is similar to that reported for chromates and dichromates in aqueous solutions.21, 23, 57 These data provide no insight on whether the monomers are adsorbed or reduced at the surface. The mechanisms of inhibition by  will be discussed in a future communication. However, it is important to note that the efficacy of vanadate inhibition seems to reach a limiting value in the same range of
will be discussed in a future communication. However, it is important to note that the efficacy of vanadate inhibition seems to reach a limiting value in the same range of  concentrations where the
concentrations where the  concentration reaches a limiting value (Fig. 5).
concentration reaches a limiting value (Fig. 5).
A comparison of the long-term performance of orange decavanadate and clear metavanadate solutions was carried out by analyzing polarization resistance values,  , extracted by LPT and by optical inspection after OCP exposure. Samples of AA2024-T3 were exposed to aerated pH 8.71 solutions containing
, extracted by LPT and by optical inspection after OCP exposure. Samples of AA2024-T3 were exposed to aerated pH 8.71 solutions containing  with or without vanadates as indicated. Typical results are shown in Fig. 7. In the absence of inhibitor,
with or without vanadates as indicated. Typical results are shown in Fig. 7. In the absence of inhibitor,  varied between 5 and
varied between 5 and  , and samples were severely pitted after the
, and samples were severely pitted after the  experiment. The fluctuation of
experiment. The fluctuation of  in the solution without inhibitor is probably associated with the pitting corrosion. An orange decavanadate solution containing
in the solution without inhibitor is probably associated with the pitting corrosion. An orange decavanadate solution containing  (present in a solution that was initially
(present in a solution that was initially  ) exhibited
) exhibited  values that fluctuated between 50 and
values that fluctuated between 50 and  initially. However, after about
initially. However, after about  ,
,  decreased with time to
decreased with time to  . In contrast, in a clear metavanadate solution containing
. In contrast, in a clear metavanadate solution containing  , significantly higher
, significantly higher  values were observed. Polarization resistance typically varied between 300 and
values were observed. Polarization resistance typically varied between 300 and  and was virtually independent of
and was virtually independent of  concentration above
concentration above  . The low corrosion rate was sustained during the
. The low corrosion rate was sustained during the  of exposure. Samples exposed at OCP to orange decavanadate solutions containing
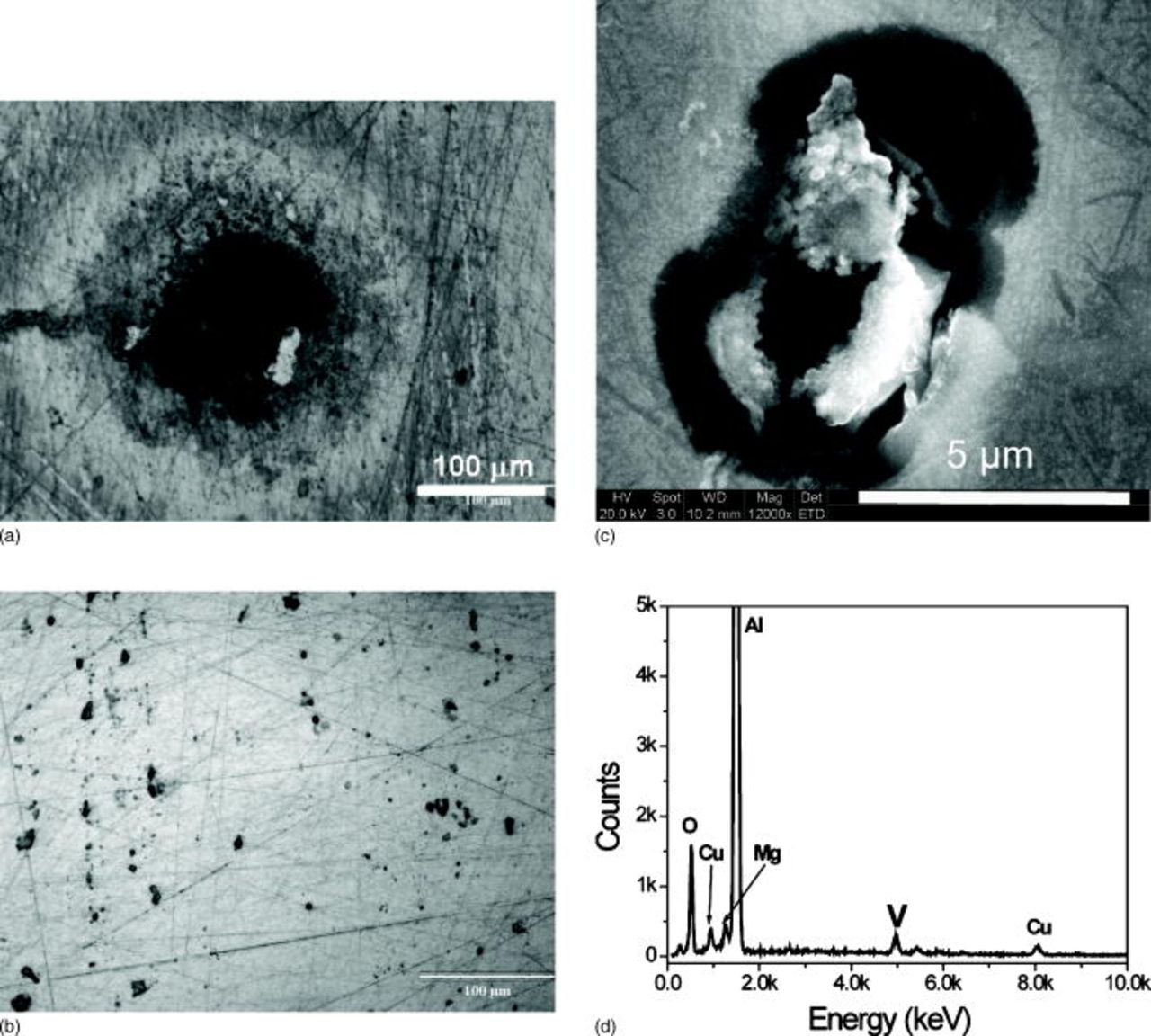
of exposure. Samples exposed at OCP to orange decavanadate solutions containing  rapidly developed pits that could be observed easily with the unaided eye. Figure 8a is an optical micrograph of an AA2024-T3 after 14 days in an orange decavanadate
rapidly developed pits that could be observed easily with the unaided eye. Figure 8a is an optical micrograph of an AA2024-T3 after 14 days in an orange decavanadate  chloride solution with initial total vanadate concentration of
chloride solution with initial total vanadate concentration of  . Very large pits at the surface are evident. In contrast, only small pits were observed after exposure to clear metavanadate
. Very large pits at the surface are evident. In contrast, only small pits were observed after exposure to clear metavanadate  chloride solutions (Fig. 8b). Interestingly, in both cases, pits were always covered by a corrosion product that was enriched in vanadium as shown by EDS (Fig. 8c). The scratches observed in Fig. 8c and d are common for AA2024-T3 polished to
chloride solutions (Fig. 8b). Interestingly, in both cases, pits were always covered by a corrosion product that was enriched in vanadium as shown by EDS (Fig. 8c). The scratches observed in Fig. 8c and d are common for AA2024-T3 polished to  diamond paste. Atomic force microscopy measurements revealed that the scratches are less than
diamond paste. Atomic force microscopy measurements revealed that the scratches are less than  deep. Similar results were obtained for samples only polished to 1200 grit.
deep. Similar results were obtained for samples only polished to 1200 grit.
Figure 7. Polarization resistance of AA2024-T3 in  solutions containing no inhibitor,
solutions containing no inhibitor,  or
or  .
.
Figure 8. Optical micrographs of AA2024-T3 after 14 days of exposure to aerated  containing (a)
containing (a)  and (b)
and (b)  . (c) Secondary electron image of a small pit nucleated after 14 days exposure to
. (c) Secondary electron image of a small pit nucleated after 14 days exposure to  , and EDS analysis of the corrosion products showing enrichment in vanadium.
, and EDS analysis of the corrosion products showing enrichment in vanadium.
Since high-pH orange decavanadate solutions are not at thermodynamic equilibrium, it was of interest to analyze the effects of solution aging on corrosion inhibition performance. A  orange decavanadate solution adjusted to pH 8.71 was aged for 10 days. Samples were extracted for
orange decavanadate solution adjusted to pH 8.71 was aged for 10 days. Samples were extracted for  NMR analysis and electrochemical characterization after 1, 3, and 10 days. The solution pH decreased slowly over time; therefore, pH was readjusted to 8.71 before the experiments.
NMR analysis and electrochemical characterization after 1, 3, and 10 days. The solution pH decreased slowly over time; therefore, pH was readjusted to 8.71 before the experiments.
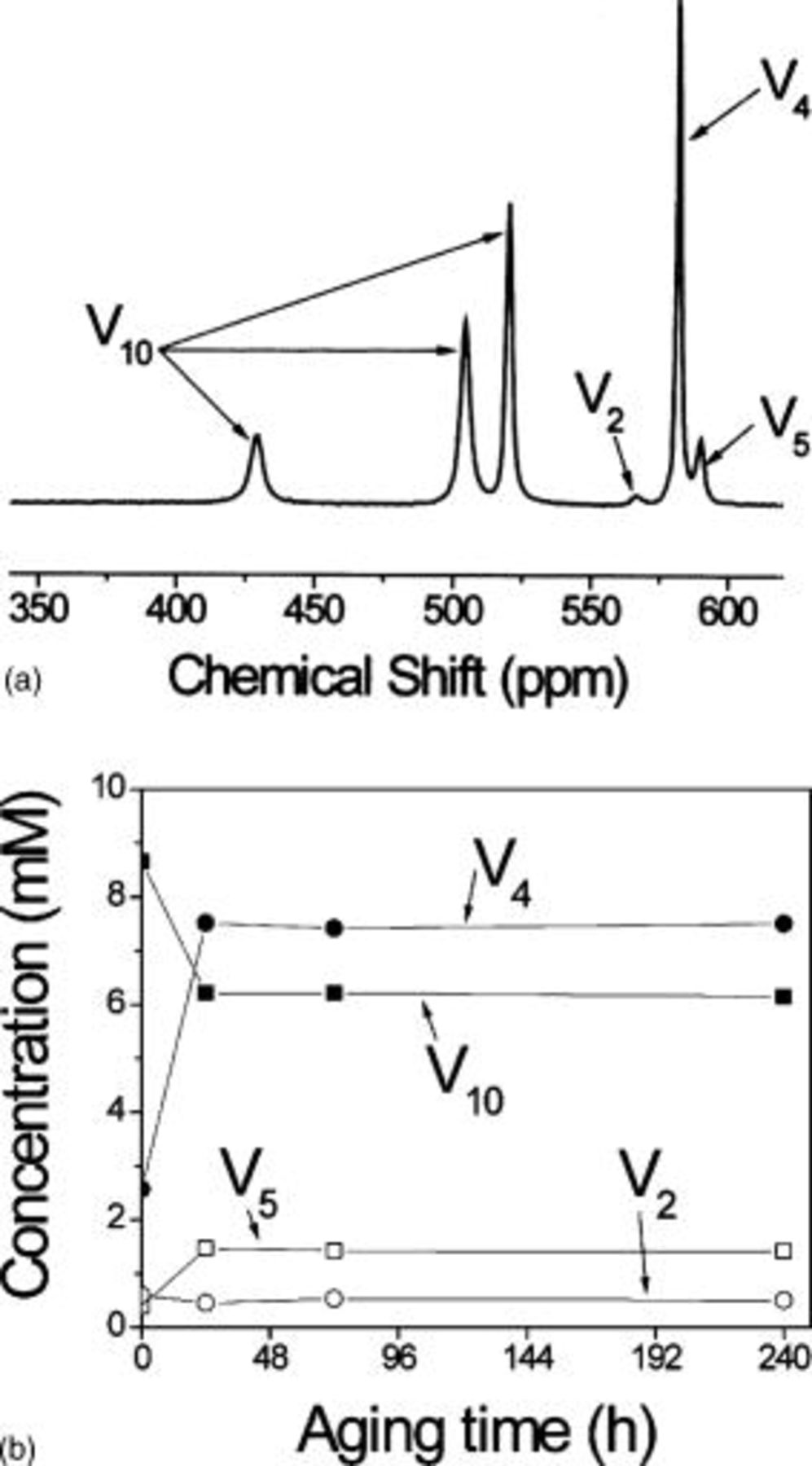
The  NMR spectrum shows that after
NMR spectrum shows that after  aging the amount of
aging the amount of  was reduced and
was reduced and  became predominant (Fig. 9a). However, decavanadate was still present and monovanadate did not form during aging, indicating that the depolymerization to form
became predominant (Fig. 9a). However, decavanadate was still present and monovanadate did not form during aging, indicating that the depolymerization to form  is not favored under these conditions. After 3 and 10 days no significant changes in speciation were observed (Fig. 9b). This suggests that the rate of depolymerization is extremely slow. Samples without pH readjustment were also analyzed for comparison. Except for small changes in δ, no significant differences in speciation were found. Consequently, from a practical standpoint, polymerization of
is not favored under these conditions. After 3 and 10 days no significant changes in speciation were observed (Fig. 9b). This suggests that the rate of depolymerization is extremely slow. Samples without pH readjustment were also analyzed for comparison. Except for small changes in δ, no significant differences in speciation were found. Consequently, from a practical standpoint, polymerization of  is not completely reversible.
is not completely reversible.
Figure 9. Effect of aging. (a)  NMR spectrum of a
NMR spectrum of a  orange decavanadate solution aged
orange decavanadate solution aged  and (b) concentration of the different oligomers as a function of aging time.
and (b) concentration of the different oligomers as a function of aging time.
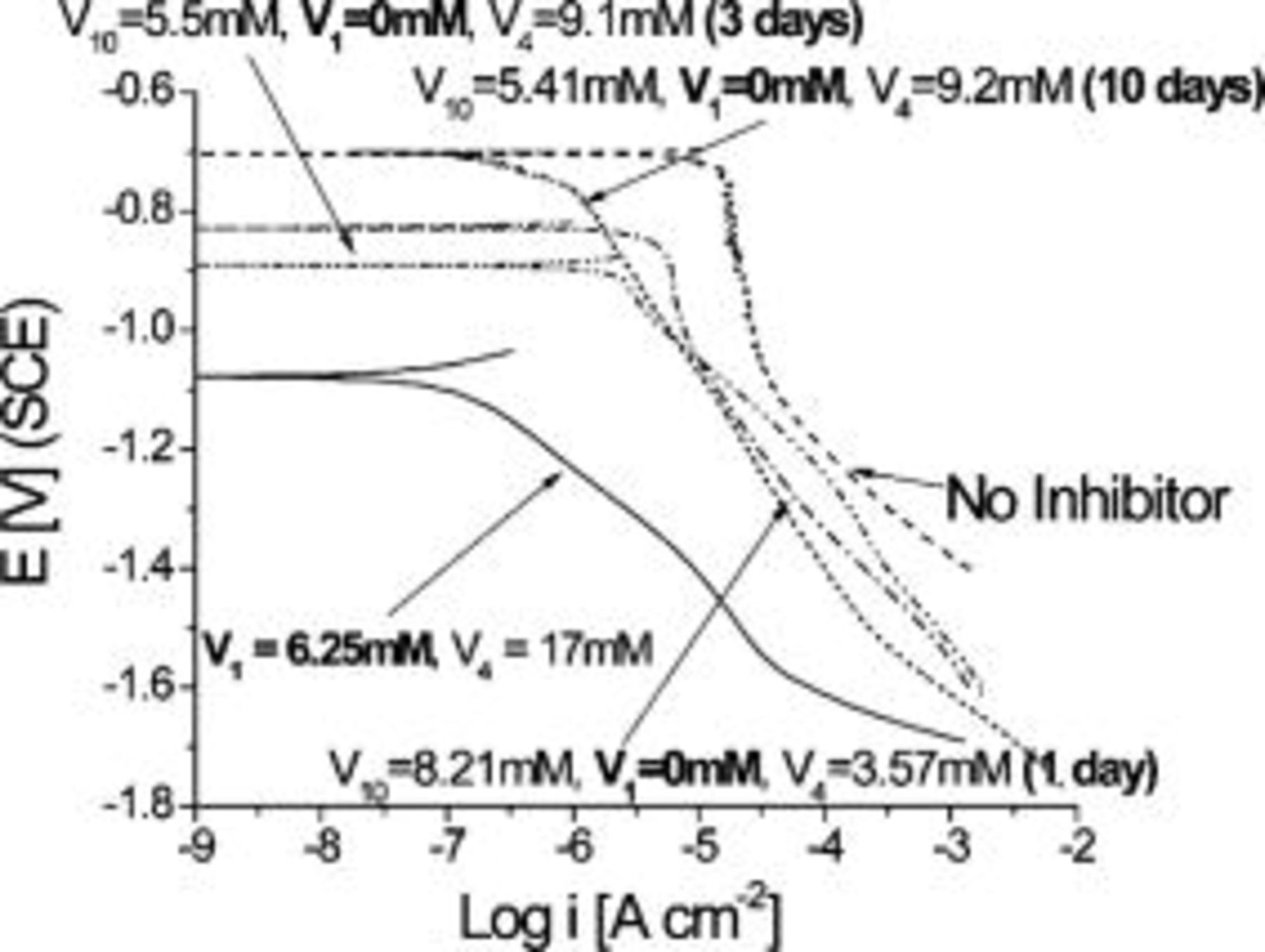
Cathodic polarization curves did not show significant variations with aging time of the orange decavanadate solutions (Fig. 10). A large limiting current associated with a relatively fast rate of ORR was observed. Similarly, the OCP was  more positive than the OCP in clear metavanadate solutions. It is interesting that polarization curves in metavanadate solutions were very reproducible, but the scatter for the orange decavanadate solutions was large. The polarization plots shown here represent a lower limit for decavanadate solutions to present conservative results. In many cases currents as large as the limiting current in solutions with no inhibitor added were obtained. Since the main consequence of aging was the rapid formation of
more positive than the OCP in clear metavanadate solutions. It is interesting that polarization curves in metavanadate solutions were very reproducible, but the scatter for the orange decavanadate solutions was large. The polarization plots shown here represent a lower limit for decavanadate solutions to present conservative results. In many cases currents as large as the limiting current in solutions with no inhibitor added were obtained. Since the main consequence of aging was the rapid formation of  species, these findings indicate that inhibition of ORR is not imparted by
species, these findings indicate that inhibition of ORR is not imparted by  or by
or by  . In other words, it seems plausible that the higher molecular weight oligomers do not impart good protection since they cannot lower the rate of
. In other words, it seems plausible that the higher molecular weight oligomers do not impart good protection since they cannot lower the rate of  reduction.
reduction.
Figure 10. Effects of solution aging on cathodic behavior of AA2024-T3 in orange decavanadate solutions.
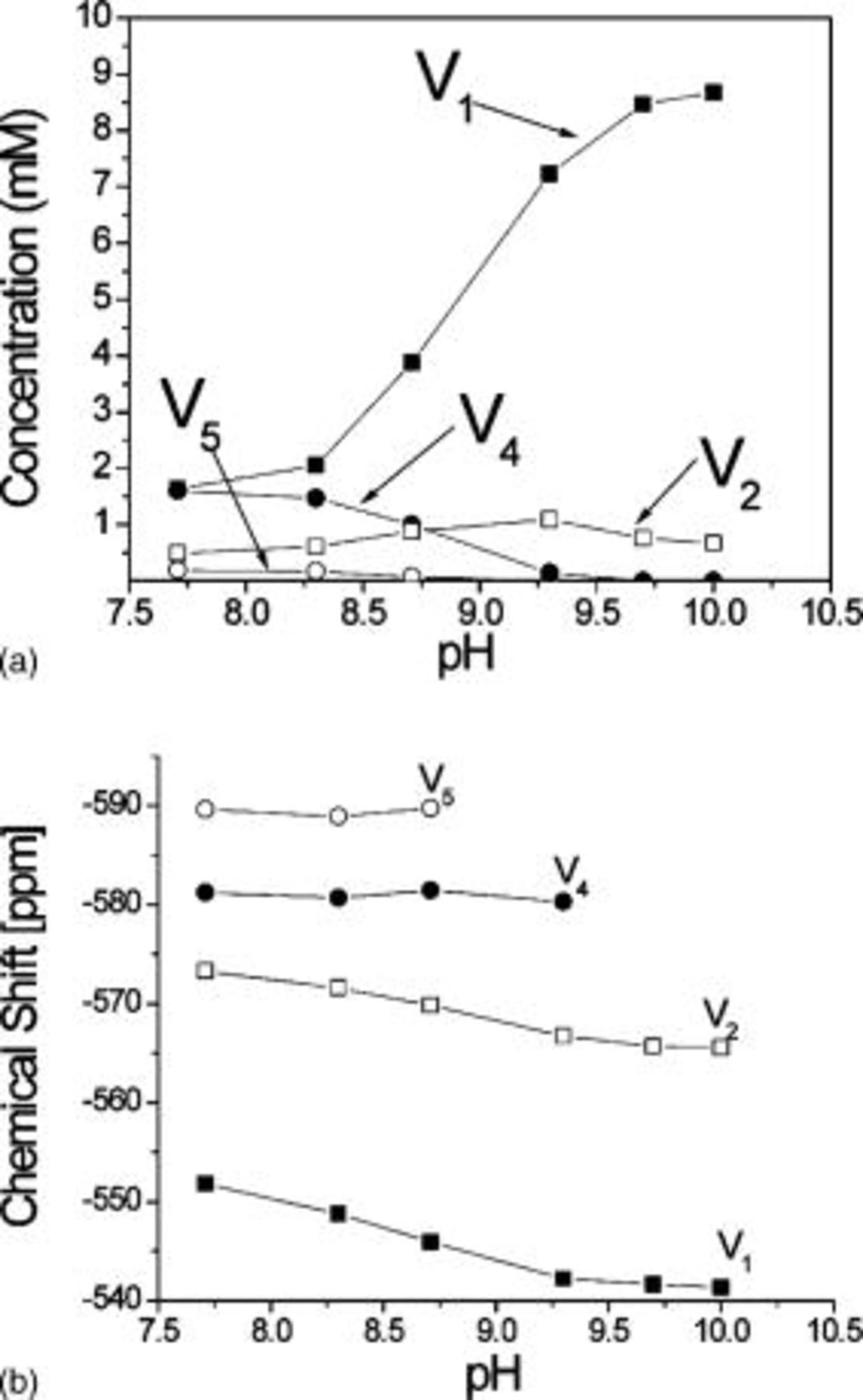
The effects of incremental pH on speciation of  clear metavanadate solutions were also investigated by
clear metavanadate solutions were also investigated by  NMR. Figure 11a summarizes the variation of the concentration of the different oligomers as a function of pH. As pH increased,
NMR. Figure 11a summarizes the variation of the concentration of the different oligomers as a function of pH. As pH increased,  rapidly became predominant and
rapidly became predominant and  and
and  peaks diminished. Above pH 9 only signals from
peaks diminished. Above pH 9 only signals from  and
and  were obtained. Chemical shift vs pH is plotted in Fig. 11b. In good agreement with Heath et al. and Larson,42, 44 the equilibrium for both
were obtained. Chemical shift vs pH is plotted in Fig. 11b. In good agreement with Heath et al. and Larson,42, 44 the equilibrium for both  and
and  shifted toward the deprotonated form at
shifted toward the deprotonated form at  . Chemical shift of the higher molecular weight oligomers is virtually independent of pH.32, 42, 44
. Chemical shift of the higher molecular weight oligomers is virtually independent of pH.32, 42, 44
Figure 11. Effect of pH. (a) Concentration of the oligomers present in  metavanadate solutions as a function of pH and (b) chemical shift as a function of pH. Above pH 9 signals only from
metavanadate solutions as a function of pH and (b) chemical shift as a function of pH. Above pH 9 signals only from  and
and  are observed.
are observed.
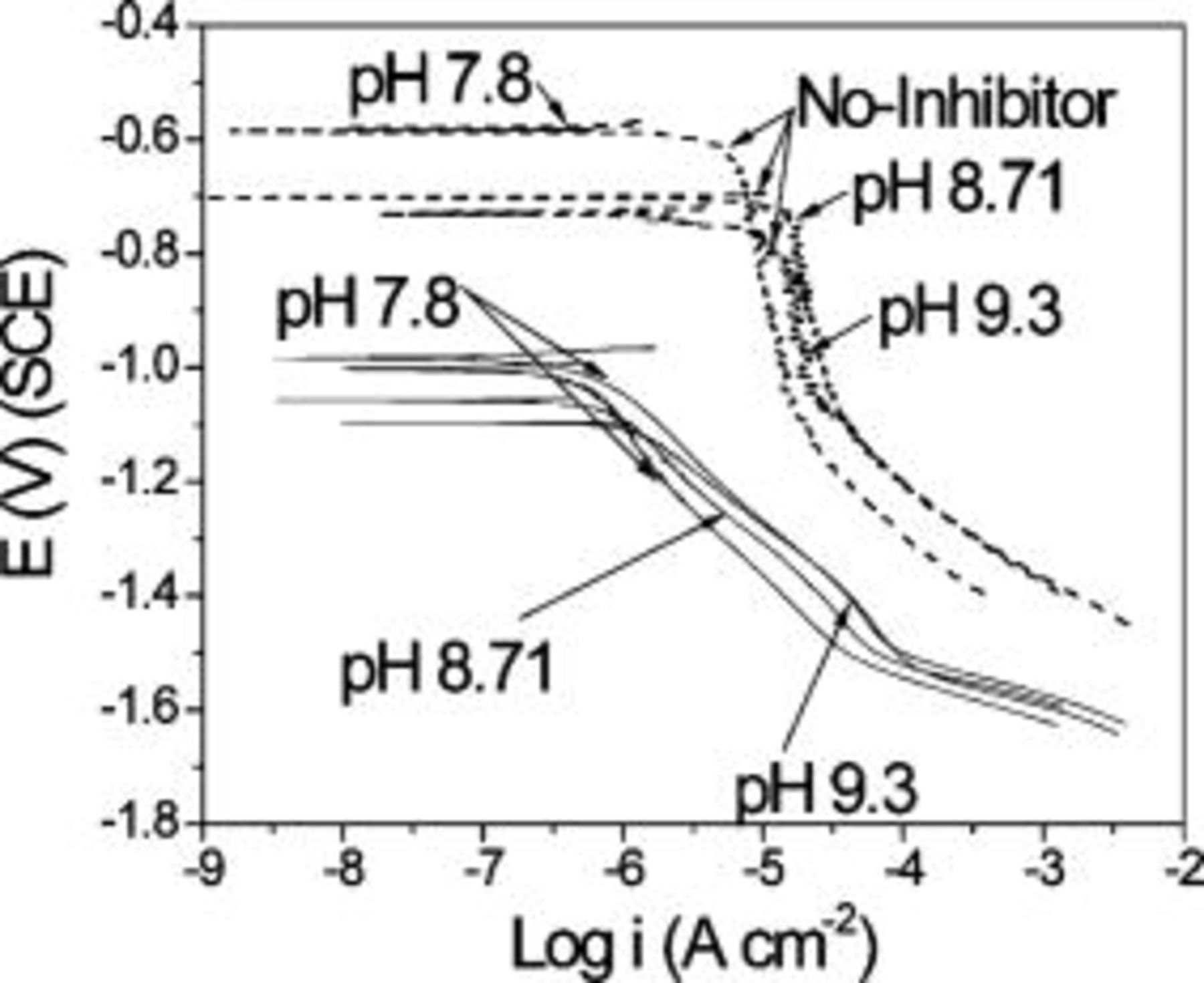
Taking into account these findings, it is possible to analyze the effects of  concentration, protonation state, and pH on inhibition. Cathodic polarization experiments in 0.5 M NaCl + 10 mM NaVO3 with different pH were carried out and compared with solutions containing no inhibitor at the same pHs. The exact concentration of
concentration, protonation state, and pH on inhibition. Cathodic polarization experiments in 0.5 M NaCl + 10 mM NaVO3 with different pH were carried out and compared with solutions containing no inhibitor at the same pHs. The exact concentration of  and
and  can be extracted from the
can be extracted from the  NMR data (Fig. 11a).
NMR data (Fig. 11a).
The polarization curves in the solutions with no inhibitor were identical except for a change in the OCP. All polarization curves in the metavanadate-containing solutions exhibited much lower ORR rates, and there was no clear influence of pH (and thus  concentration) (Fig. 12). This suggests that above a critical concentration, most of the local cathodes are blocked by
concentration) (Fig. 12). This suggests that above a critical concentration, most of the local cathodes are blocked by  (or a combination of
(or a combination of  and
and  ), which greatly reduces the rate of oxygen reduction.
), which greatly reduces the rate of oxygen reduction.
Figure 12. Cathodic polarization curves of AA2024-T3 exposed to  at high pH with no inhibitor or clear metavanadate.
at high pH with no inhibitor or clear metavanadate.
These findings should directly impact the development of new coating systems based on vanadates. To date, most of the work with vanadium compounds was focused on hosting decavanadates in hydrotalcite pigments or in conversion coatings.14, 26, 30 Since monovanadate appears to be the strongest corrosion inhibitor of the system, any coating scheme based on vanadium should release  . The electrolyte developed in atmospheric exposure of aircrafts is thought to be neutral to basic,59 which would impede the formation of decavanadate. Special care should also be taken during coating formulation. Since
. The electrolyte developed in atmospheric exposure of aircrafts is thought to be neutral to basic,59 which would impede the formation of decavanadate. Special care should also be taken during coating formulation. Since  triggers decavanadate formation, the pH of the coating bath has to remain above that critical level. The main problem of
triggers decavanadate formation, the pH of the coating bath has to remain above that critical level. The main problem of  as pigment is its relatively large solubility, which would end up producing blistering after water uptake. However, Smith et al.28, 60 and Nazarov et al.61 produced a variety of vanadate pigments with a lower solubility than
as pigment is its relatively large solubility, which would end up producing blistering after water uptake. However, Smith et al.28, 60 and Nazarov et al.61 produced a variety of vanadate pigments with a lower solubility than  , by simply reacting
, by simply reacting  with different metallic chlorides such as
with different metallic chlorides such as  and
and  . This suggests that it could be possible to have control over the release kinetics.
. This suggests that it could be possible to have control over the release kinetics.
In addition to the effects of vanadates on the ORR, the effects of both clear metavanadate and orange decavanadate solutions on the anodic reaction were studied in detail and are the topic of a future communication. However, the effects of clear metavanadate solution on the anodic reaction were significantly smaller than the large reduction in the rate of the ORR.
Finally, it is unclear what would happen inside an acidic crevice. However, the absence of crevice attack under masked regions during long-term exposure seems to indicate that the blockage of the local cathodes by monovanadates diminishes any possible anodic dissolution, including crevice corrosion. Nevertheless, a more detailed investigation is still required.
Conclusions
Speciation of vanadate solutions with varying concentrations and pH was studied by  NMR spectroscopy and the effects of corrosion inhibition for AA2024-T3 were investigated. The following conclusions can be obtained:
NMR spectroscopy and the effects of corrosion inhibition for AA2024-T3 were investigated. The following conclusions can be obtained:
1. Decavanadate only formed when metavanadate was added to solutions of pH 3 or less. The final pH after vanadate addition was independent of the initial pH for initial pH of 3 or greater. Therefore, it is impossible to change the pH of a metavanadate solution without forming decavanadates.
2. Acidification of clear metavanadate solutions to pH 4 or lower polymerized all the oligomers to form  . Readjusting the pH to 8.71 produced a partial depolymerization of
. Readjusting the pH to 8.71 produced a partial depolymerization of  to form
to form  ,
,  , and
, and  . Orange solutions always contained decavanadates and never contained monovanadates.
. Orange solutions always contained decavanadates and never contained monovanadates.
3. Orange decavanadate solutions containing  exhibited no significant inhibition of the ORR and increasing decavanadate concentration was detrimental. Aging of the metastable decavanadate solutions for up to 10 days did not improve protection.
exhibited no significant inhibition of the ORR and increasing decavanadate concentration was detrimental. Aging of the metastable decavanadate solutions for up to 10 days did not improve protection.
4. Clear metavanadate solutions containing monovanadate exhibited strong inhibition of the ORR, to a level similar to chromate. At a fixed pH, increased  concentration in clear metavanadate solutions increased inhibition efficiency.
concentration in clear metavanadate solutions increased inhibition efficiency.
5. Since the orange decavanadate solutions contain no  and the clear metavanadate solutions contain no
and the clear metavanadate solutions contain no  , these results suggest that decavanadates are detrimental or ineffective inhibitors and monovanadates provide the best inhibition.
, these results suggest that decavanadates are detrimental or ineffective inhibitors and monovanadates provide the best inhibition.
6. As the pH of clear metavanadate solutions increases,  and
and  become the predominant species, but improvement in inhibition was not found.
become the predominant species, but improvement in inhibition was not found.
7. A coating system based on vanadates should be able to release monovandates. The formation of decavanadates during coating formulation should be avoided.
Acknowledgments
This work was partially funded by AFOSR under award F 49620-02-0321, Major J. Gresham, Ph.D., contract monitor.
The Ohio State University assisted in meeting the publication costs of this article.