Abstract
Magnesium-sulfur batteries have developed as a new and emerging technology benefiting from high energy density, low cost, reasonable safety, and excellent energy storage due to the high natural abundance of electrochemically active materials and low dendrite formation in magnesium. Here we report various enhancement strategies and also focus on using carbon electrodes, coating layers of carbon over the cathodes, carbon nanotubes, reduced graphene oxide, graphene-carbon nanotubes in magnesium-sulfur batteries because of its high conductivity and improved overall electrochemical functioning of the magnesium-sulfur battery. However, developing these batteries remains challenging due to significant problems caused during theirs operation, such as self-discharge, Mg-anode passivation, insufficient reversible capacity, low sulfur cathode utilization, and rapid capacity loss. We acknowledge the synthesis of non-nucleophilic electrolytes, both situ characterizations of anode or electrode reactions and kinetics, strategic development of sulfur-based cathodes and carbon electrode in Mg–S battery as a critical factor toward improvement in cycle performance, specific capacity, overpotential and working voltage, and confinement of Mg-PS polysulfide, to limit the shuttling of polysulphides, steady accumulation and desolvation of magnesium divalent ions to create a magnesium-conducting surface electrode interphase(SEI). We also present a detailed description of the Mg–S battery, its challenges, future research directions for the practical implementation of the various developed electrolyte and electrodes.
Export citation and abstract BibTeX RIS
Nowadays, thriving developments are taking place in all the fields, so portable electronic devices, innovative grid applications, electric vehicles, and wearable devices are growing. As a result, energy storage devices requirements are increasing. A good battery should have naturally abundant raw materials, promising cycling stability, high energy density, cheap, and most importantly, high safety. Li-ion is the most advanced battery among all the fuel cells, and this was the first battery to be commercialized and used for practical applications. They are used extensively in our day-to-day lives of electronic devices like mobile phones, laptops, emergency power backup systems, etc. However, these batteries could not be used as a source of power in electric vehicles since they could not deliver an adequate amount of energy, and the availability of resources that are required for constructing batteries such as Li, Co, Ni is limited, which hindered the development and use of Li-ion batteries to some extent. So there is a need to find battery chemistry with low cost and abundant material which fulfills all the merits of a perfect storage device. Therefore, development in rechargeable metal sulfur batteries started because this is an excellent power source that can store a large amount of energy. The concept of RMSBs is similar to any other battery, but they differ in the materials chosen for the cathode. In these batteries, the cathode is sulfur which is a non-metal. Sulfur is chosen as cathode because it is one of the most abundantly available and lightweight materials. It is also less expensive and non-toxic, which makes it a safe cathode material.
Reducing sulfur in a battery's operation can give a capacity as high as 1672 mAh per gram of active material utilized. Therefore sulfur is used as a cathode material with Lithium metal anode. Lithium-sulfur batteries replaced the Li-ion batteries for a very long time because Li/S batteries can provide an elevated theoretical energy density of approximately 2800 Wh l−1. However, several issues like the availability of Li metal, safety concern associated with the dendritic formation on lithium metal, shuttle effect, low coulombic efficiency, and low cyclic stability hinders Li-S battery use at a vast scale. In contrast, Li anode Mg is a highly safe,
naturally abundant, cheaper, chemically inert anode material with a high theoretical volumetric capacity of 3832 mAh cm−3. Hence, when magnesium with sulfur cathode can provide a relatively higher theoretical energy density of 1684 Wh Kg−1, Smooth surfaces are favored by the Mg metal compared to Na and Li because it has a highly coordinated configuration and low diffusion barriers. Due to safety concerns, Li batteries required intercalation electrodes; however, Mg metal can be straightforwardly utilized as solid anode material in Mg–S cells. Also, the divalent nature of magnesium ions stores two electrons and provides greater capacity. Due to this, they find their use in electric vehicles. The electro- deposition and stripping of magnesium batteries are dendrite-free. Also, the low reduction potential of magnesium makes it more utilitarian. There are many reports on the increasing use of sodium-sulfur batteries because it works at room temperature, but Na metal is not as abundant and safe as Mg.
In constructing rechargeable magnesium (Mg) batteries, the most critical challenge is forming a magnesium electrolyte. Properties of cathode and battery performance are hugely dependent on the properties of the electrolyte. Mg batteries presented improved cycling properties, and Mg electrodes showed highly reversible behavior when a family of electrolytes based on Organo-halo-aluminate and Magnesium salt in tetrahydrofuran (THF) was used.
The stability of carbon is remarkable and superior to others in most electrochemical environments. Carbon cloth has a three-dimensional porous structure that paves a pathway to exceptional electronic properties and improves the ionic conductivity by promoting the accessibility of electrolytes into the pores. However, since the nature of carbon cloth is hydrophobic, the magnesium and polysulphide interlinkage and reaction are weak, leading to the leaking of some intermediate products into the electrolyte.
Despite having many advantages, several issues need to be addressed. Some of the issues faced are low sulfur utilization in the cathode during the working of battery, sensitive overcharging nature, and generation of polysulphides, and sluggish diffusion of magnesium ions, all of these result in substandard electrochemical performance. Another challenge is the evolution and advancement of an appropriate electrolyte with high ionic conductivity and enables reversible deposition of Mg. It is because of the powerful nature of sulfur that it is electrophilic, which requires non-nucleophilic electrolytes. Considerable efforts are being made to augment battery performance and alter electrode materials, separators, and the amalgamation of revolutionary electrolyte structures, leading to improved battery performance.
Understanding Mg–S battery
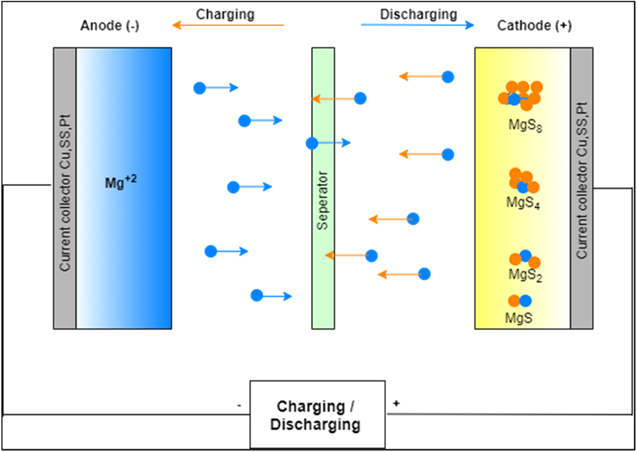
Rechargeable magnesium sulfur batteries have been chosen as an electrochemical power accumulator device because Mg has a high capacity (3,832 mAh cm−3), significantly less reduction potential, naturally abundant, operational safety, 1 and Mg anodes are highly reversible. The Mg anode material is considered the safe electrode in liquid electrolytes because it does not form dendrites on the electrode surface. Mg favors the expansion of smooth surfaces because of its highly coordinated configuration and lower diffusion barriers. As compare to other anode metals, Mg is economical, and compounds of Mg are non-toxic. Sulfur theoretical capacity is also high (3, 459 mAh cm−3 or 1,671 mAh g−1 ). 2 So together, the Mg–S battery has high discharge capacity,coulombic efficiency, and cyclic reversibility. Figure 1 shows the working of an Mg–S battery, the charging/discharging processes, and its components.
Figure 1. Mg–S battery is an electrochemical cell in this negative electrode is an anode made up of Mg metal contain Mg+2 ion, and the positive electrode is the cathode, made of carbon with sulfur-doped in pores and release S-2 ions. In discharging process, various polysulphides are formed on the cathode surface because of the variable valency of Sulphur (S8).
Download figure:
Standard image High-resolution imageAn Mg–S battery binder should be non-toxic, inexpensive, have good bond strength, have less electrolyte absorption, and be highly soluble in solvents. Electrodes are commonly the product of carbon host fabric, so this desires to have a robust chemical anchoring of sulfur, excessive electric conductivity, a robotically solid framework, tiny pores without large openings, easy access to a liquid electrolyte, etc. The major disadvantage of Mg is its more positive reduction potential, sulfur's electrophilic solid nature, and the electrical insulating property. These create problems in the advancement of the Mg–S battery.
Design & construction of Mg–S battery
Mg–S battery work at the electrochemical transformation of sulfur (S8) to magnesium sulfide (MgS). These reactions occur during the discharging process. Opposite of these reactions occur in the process of charging. 3
At negative electrode:
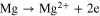
At positive electrode:
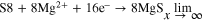
But along with these reactions some side reactions also takes place which forms polysulphides:
Step 1. (solid-liquid two-phase reaction): S8(solid) + 4e− + 2Mg2+ → 2MgS4(liquid)
Step 2. (liquid-solid two-phase reaction): MgS4(liquid) + 2e− + Mg2+ → 2MgS2(solid)
Step 3. (solid-state reaction): MgS2(solid) + 2e− + Mg2+ → 2MgS(solid) Some of the problems occur in the Mg–S battery:
In the discharge process, low-order polysulfide forms and precipitates at the sulfur cathode. In the solid phase, magnetization slows down the conversion of long-chain polysulfides to MgS. The nature of polysulfides like Mg3S8, MgS2, or MgS is insulating, alongside the lower diffusivity of Mg2+ within solid phase, leading to massive overpotential on the last step of discharge likewise final products re-conversion to long-chain polysulfide in charging process is lacking. 3 Dissolution of polysulfides in the electrolyte is another problem. Passivation of magnesium metal anode occurs in most Mg–S batteries; therefore, the localized or inhomogeneity increase of Mg deposits, especially at excessive current densities, is the issue associated with the Mg anode passivation. After considering all these issues, the design of the Mg–S battery is done. Figure 2 depicts the various essential components and some additional components in the design and successful operation of the Mg–S battery.
Figure 2. Changes required while designing the components of the battery to improve the performance of the Mg–S battery.
Download figure:
Standard image High-resolution imageThe high theoretical energy density and natural abundance of sulphur in Mg–S batteries are considered to be a great substitute for high-energy rechargeable energy storage devices. However, the issues including insulation of sulphur, formation of polysulfides, their dissolution which results in short cycle lifespan have prevented Mg–S battery from being used in practical applications. Here we have categorised various physical and chemical methods to improve the effectiveness and performance of the battery. Through surface coating the shuttle effect in Mg–S batteries can be suppressed. Carbon has a high electrical conductivity, elastic nature and dense structure which makes it a remarkable coating material in enhancing the electrochemical performances of various electrode materials that are used in battery. A solvent system containing of a mixture of tetraethylene glycol dimethyl ether (TEGDME)/diethylene glycol dimethyl ether (DEGDME) with commonly used additives such as LiNO3 when added to Mg−S batteries can increase their cycling stability and reduce the polysulfide shuttle. An artificial SEI between the Magnesium anode and separator which reduces the number of side reactions on the metal surface is an appropriate strategy to mitigate the self-discharge and various negative effects of the polysulfide shuttle in the battery. The barriers for polysulfide physical separation i.e. use of separators such as carbonaceous materials. Many porous carbon materials, such as microporous and mesoporous carbon have the ability of adsorbing polysulfides into their large surface area and abundant pores structure. Many other separators are also mention while discussing the cathode material preparation in this study. Sulphur loading is another process through which sulphur can be introduced into the fabricated matrixes containing enough pores for the accommodation of sulphur. Melt diffusion gives a closer contact between sulphur and matrixes, vapour deposition is another approach to obtain cathodes that can easily accommodate sulphur molecules. Both these methods are highly efficient in preparing composite cathodes with carbon present in them though carbon and sulphur bonding is strong but it also facilitates the sulphur particles into the porous voids while the matrix consists of oxygen-rich carbon. Many carbon structures have been described further in the report that are good matrix to host sulphur such as graphene, porous carbon, CNTs, mesoporous carbon and graphene, nanofibers, a hollow carbon fibers foam which consists of multiwalled carbon nanotube and carbon black. The host exhibited higher absorbability and fixation of polysulfides and also showed improved electrochemical performance keeping good cycling stability with sulphur loadings. Doping carbon with various elements like boron, nitrogen, phosphorus, or sulphur can be done to enhance the chemical adsorption towards polysulfides without affecting the conductivity. Apart from this, many nano-sized metal oxides structures can be used to fabricate feasible cathodes. Materials containing porous and hollow structures can provide physical confinement and enhance adsorption of polysulfides. Many sulfurized polymers were also design to improve the physical adsorption of sulphur particles. Thus composite electrodes with various chemical and physical confinement ways can be a promising approach to reduce the shuttle effect of polysulfides. The cathode design can ideally consist of higher sulphur loading capacity, conductivity of material, larger specific surface area and high adsorption sites such as porous or hollow structures. 4
A battery consists of mainly three components: cathode, anode, and electrolyte. Different types and forms of anode, cathode, and electrolyte can be used according to the need. Other additional materials are added to these three components in order to enhance the battery performance. These materials affect the performance of the battery directly or indirectly. Various components help in improving distinct properties of electrodes and electrolytes. An anode is made up of magnesium material for an Mg–S battery, and a cathode is made up of porous carbonaceous sulfur-doped material. The main reason for capacity fading in reversible magnesium batteries is the dissolution of sulfur in electrolytes. This can be prevented by using a non-nucleophilic electrolyte.
Cathode
This consists of the carbon host material, binder, and current collector. The porous carbon substances benefit from increasing the sulfur cathode overall performance, including excessive electronic conductivity, powerful polysulfide confinement, and excessive surface-area-to-volume ratio. Carbon as a host material can also reduce the damaging impact of volumetric enlargement during cell cycling. Various types of carbon host material like activated carbon cloth, multiwalled carbon nanotubes, hollow carbon spheres, mesoporous and microporous carbon, reduced graphene oxide, N-doped graphene, and carbon black can be used further explanation on this will be given in section 3. The sulfur-microporous carbon composite is an appealing cathode fabric because of its super functionality of stopping higher-order polysulfide dissolution. These little sulfur S2–4 allotropes are confined inside the micropores. A cell is coated with TiS2 (0.15 mg cm−2) to improve the capacity because TiS2 is a promising positive electrode intercalation material. 5 Besides the sulfur and carbon hosts material chemical interaction, the dissolution of a few polysulfides inside the electrolyte is challenging to avoid, and hence the polysulfide shuttle effect at the Mg metal anode will occur. Some sulfur cathodes are also made to reduce polysulfide species mobility inside the cathode by forming a covalent bond or high chemical absorption between the cathode hosts and sulfur species. This strong covalent bond is formed due to the reaction of sulfur with graphdiyne. Therefore, the sulfur molecules are nicely constrained in the triangle-shaped pores within graphdiyne.
Current collector
It is a crucial element of the battery. This performs a key function inside the overall performance enhancement of the Magnesium sulfur battery. For example, several current collectors, Cu, Pt, Al, SS, Inconel 625, etc., can be used. Most Cu current collectors are used as it gives higher discharge/charge capacity and cyclic stability. In Mg(CF3SO3)2 electrolyte with Tg + THF mixture (1:1 quantity ratio) solvent. 6 Compared to platinum electrodes, the electrolyte displays less reversibility and current densities on SS, Cu, and Al non-inert electrodes, and low anodic stability, which usually results from non-inert metals corroded by Cl− ion present inside the solution. When Stainless steel (SS) is employed as a current collector, the Mg/S battery delivers the starting l discharge capacity of 9 mAh per grams of active material, significantly less than the capacity received from the cell when a Cu current collector is used. The sulfur utilization increases due to strong interaction between copper, sulfur, and copper sulfides, 7 higher electronic conductivity. This also enhances the S cathode cycling stability in the nucleophilic electrolyte.
Binders
Binder substances are used to hold the particles of active material inside the electrode of a reversible magnesium battery together to maintain a good connection between the electrode and the contacts. These binding substances are commonly inert and play an essential role in the manufacturability of the battery. Organic-soluble poly(vinylidene fluoride) (PVDF) and water-soluble carboxymethyl cellulose (SCMC), β-cyclodextrin (β-CD), sodium polyacrylate (PAAS), locust bean gum (LBG),poly(ethylene oxide) (PEO),and guar gum (GG), 8 are generally used as the binder. Zang S. et al. (2020) compared these binders, which shows that capacity retention of the sodium polyacrylate(PAAS) binder is much better than other binders. The PAAS binder performs a vital function in reaching a higher uniformity, tremendous cohesion, and more potent binding ability for the S@pPAN electrode. For the PAAS binder, the electrode's charge−discharge process's voltage polarization decreases, which improves cycling stability and rate capability. The formation of an artificial solid-electrolyte-interface (SEI) film at the electrodes also PAAS performs a major role. 9
Anode
This comprises different forms of Mg metal like pressed Mg anode made up of Mg powder or alloy compounds or Mg foil. To enhance the performance of Mg metal anode, Mg metal anodes with a high surface area are used in Mg/S battery rather than magnesium foil. 10 On ball milling of powdered magnesium with carbon black or powdered Graphite continued by pressing the pressed anode is made. Mg powder anode provides porous characteristics and, therefore, higher surface area and better soaking properties. Because of the larger surface area, Mg electrodes can react chemically and scavenge the most dynamic impurities present in the solution. A pressed magnesium metal anode cell can deliver a capacity of 500 mAhg−1 sulfur (422 mWh g−1 sulfur ) with upgraded cyclic stability. 11
Materials of Mg2+ ion insertion had been proposed as a new set of anode active substances. Many elements of the p-block M(e.g., In, P, Bi, Si, Sb, Ge, Pb, Sn, Al, Ga, etc.) are alloyed with Mg. As a result, Zintl phases Mg x M are formed. These Mg x M alloys possess low alloying potentials and excessive theoretical specific capacities due to a divalent ion of Mg existing within the Zintl phases. Alloying is done to overcome the Mg metal passivation problem. The metallic form of magnesium always passivates on the anode. Bi is a suitable magnesium alloy anode because the theoretical volumetric capacity of Bi is similar to magnesium metal (3833 mAh cm−3 ),.but if electrolyte does not contain salts of lithium, then Bi anode does not work. 3
Mg metal anode before electrochemistry or during electrochemistry modifications of the surface is done to reduce the generation of surface blocking layer at the anode. An artificial Mg2 + ion conductive interphase can be made by Mg triflate and thermal-cyclized polyacrylonitrile (cPAN) at the surface of powdered magnesium. The Mg anode is coated and is prepared by annealing a mixture of the carbon black (10 wt%), Mg metal powder (77 wt%), polyacrylonitrile (10 wt%), and Mg(CF3SO3)2 salt (3 wt%) over the SS foil under an atmosphere of argon at 300 °C by this PAN units in situ cyclization is achieved. 3 Mg metal anode coated with MgF2 (<200 nm) can be made by submerging the magnesium metal in a solution of HF. The MgF2surfacefilm function is proposed as a divalent magnesium ion conductor that will suppress the side reactions with electrolytes. The solid electrolyte interface(SEI) is the most vital component of Mg anodes because the important reactions arise at SEI, such as dendrite formation and ion transport.SEI will protect the Mg metal. 11
Electrolytes
Electrolyte is a crucial part of a battery that acts as a medium for the transfer of Mg ions between the S cathode and the Mg anode in Mg–S batteries. Many attempts have been made to outline electrolytes that can be suitable for magnesium-sulfur batteries. It should be noted that ordinarily nucleophilic electrolytes are not compatible with S due to their electrophilic nature, which causes battery failure. This new study on nucleophilic electrolytes was further discussed along with the preparation of non-nucleophilic electrolytes. Types of electrolytes used in Mg–S batteries:
- 1)Electrolyte with a high concentration of Mg/Al is compounded by TiCl4 reaction. Electrolyte synthesis reaction was preceded by room temperature.
- 2)MgCl2, AlCl3, anthracene, and (CF3SO3)2, dissolved in THF and tetraglyme (TG) under pressure at room temperature. Solutions obtained without additional purification were used as electrolytes.
- 3)Mg(TFSI)2 - This electrolyte contains magnesium bis (trifluoromethane sulfonyl)imide (Mg(TFSI)2) in dimethoxyethane, tetrahydrofuran, triethylene glycol dimethyl ether, diethylene glycol dimethyl ether, tetra-ethylene glycol dimethyl ether. The most typical electrolyte used for electrochemical experiments of Mg/CMK3 and Mg/PTMA cells S was 0.3 M magnesium bis (trifluoromethane sulfonyl) along with (Mg (TFSI)2) dissolved in the chemical ether chemical glyme/diglyme. Outcomes of several solvents, such as THF, acetonitrile, glyme, triglyme, tetraglyme, and diglyme in Mg stripping from the Mg electrode, were scrutinized.
- 4)(Mg2(m-Cl)3·6THF) (HMDSnAlCl4−n) (n = 1, 2)—The addition of AlCl3 upgraded the performance of HMDSMgCl. Active material crystallization can be an important process in synthesizing a non-nucleophilic electrolyte with desirable properties like cathode compatibility. This step can augment coulombic efficiency, potential stability, and much more.
- 5)Ether-based electrolytes—An essential requirement for the electrolyte applied in sulfur batteries are chemical stability, and Ethers are preferred for their ability to enhance chemical stability. The more the number of oxygen atoms, the more is the solvation ability. A cell with the PEGDME-based electrolyte evinced a capacity close to 1100 mAhg−1 of sulfur material at 0.1 C.
- 6)Mg(Tf)2- MgAl2O4-PVDF-HFP, a ceramic polymer composite electrolyte, was selected for a battery design, with a maximum performance of 4 mS cm−1, a potential window of about 3.3 V, and a transmission number of 0.66.
- 7)A rechargeable Mg–S battery using an electrolyte consisting of complex active electrochemical in situ using HMDSMgCl and AlCl3 salt. Subsequently, bisamide salt Mg (HMDS)2 was widespread using Mg (TFSI)2 salt. However, in both of the scenarios, the forced availability of chloride additives (MgCl2, AlCl3) increased the degradation of the electrolyte and compelled the use of current metal collectors that are unfit for large-scale production.
- 8)Addition of many THFPB activities for the preparation of an electrolyte based on magnesium borohydride. The primary purpose of THFPB is to significantly enhance the softness of Magnesium Borohydride in diglyme solvent due to the strong bond linking the THFPB molecule and BH4− anion. Taking the help of the strong electron-acceptor power of THFPB infused, high concentration (0.5 M, approximately fifty times more than the DGM electrolyte/Pristine Mg (BH4)2) of Mg (BH4)2 was attained. Another function of the THFPB supplement is to modify the anodic stiffness of the Mg(BH4)2 electrolyte by generating new anion and cation types as indicated by the NMR, Raman, and MS spectra. The potential power window has been increased from 1.8 V to 2.8 V compared to Mg, which makes electrolytes have the ability to test high voltages. The next activity of the THFPB supplement may result in the nucleophilicity of the newly formed anion and cation, hence making the electrolytes of Mg(BH4)2/THFPB suitable with the sulfur cathode. Therefore, we explain the first use of Mg (BH4) 2/THFPB electrolyte in magnesium-sulfur (Mg–S) batteries. These studies present a practical and explicit approach to getting rid of hurdles faced by Mg(BH4)2 based electrolytes and bring out the significant impact of active additives on unsatisfactory Mg-ion electrolyte structures.
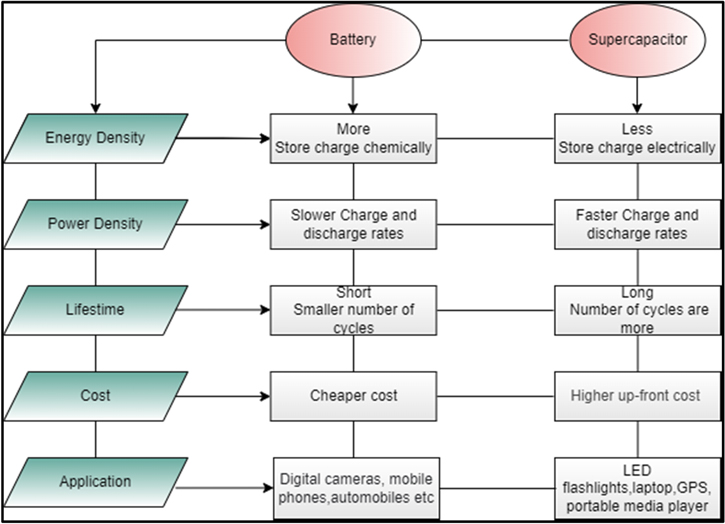
- 9)In Mg-cells, the [(HMDS)2Mg] compounds containing MgCl2 and AlCl3 in the TEG and DEG mixture were exploited. It was shown that the (HMDS)2Mg based electrolyte comprised of [HMDSAlCl3]+ [Mg2Cl3] as active electrochemical species existed as a balance in solution and no free Cl-anions. As indicated in MACC (magnesium aluminum chloride complex), an electrolytic correction process is not required for this electrolyte. The presence of MgCl2 on the anode surface after initial release, initial activation, and secondary release has been detected by EDX but may be due to contamination of Mg-based electrons, which may not be thoroughly washed. If a chlorine shuttle facing the anode were to occur, the highest value would be considered at 199. 7 eV, possessed by MgCl2, would be considered, which was not the case, and vanadium would be reduced, which was also unprecedented. Therefore, we do not exclude that Cl-shuttle occurs in the VOCl/Mg system. Fig. 3 describe the difference between battery and supercapcitor on the basis of energy density, power density, life cycle, and applications.
Figure 3. The difference between the battery and super capacitor is shown on the basis of application, cost, power and energy density.
Download figure:
Standard image High-resolution imageCurrent development in Mg–S battery
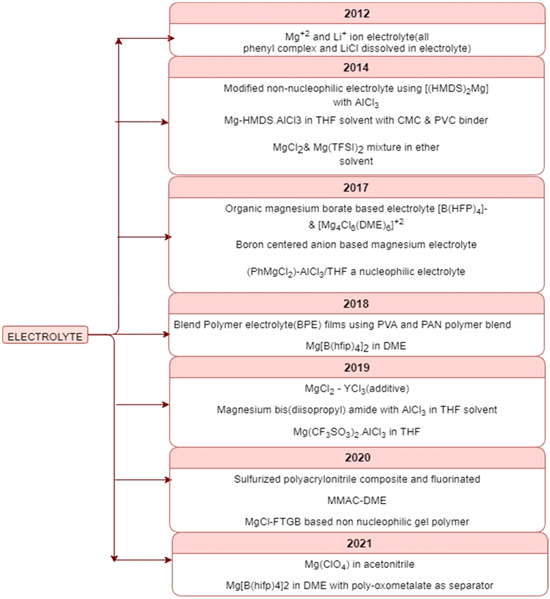
In this section, we mention some important and ground-breaking developments reported in recent years regarding Mg–S batteries. Figure 4 represents some of the technical advancements in recent years with respect to the electrolyte used in the Mg–S battery.
Figure 4. The developments in electrolytes for handling the issues faced while using the Mg-S battery.
Download figure:
Standard image High-resolution imageIn one of the earliest developments in the electrolyte, Zhao-Karger, Z. et al. (2014) reported a novel method of synthesizing nucleophilic magnesium electrolyte using a single pot two steps reaction. The reaction used magnesium bis(hexamethyldisilazane) [(HMDS) 2 Mg] and AlCl3 as reactants in various ethers. This was synthesized in different ethers or Glymes CH3O- (CH2CH2O)n-CH3 and ionic liquids. They used (HMDS) 2 Mg-based Diglyme(DEG), Tetraglyme(TEG), and their mixtures in ionic liquids represented as DEGIL and TEGIL electrolytes in their Mg–S batteries. These reactants used by them produced magnesium complexes that were non-nucleophilic. They used sulfur-carbon composite S/CMK400PEG and S/CMK160PEG as a cathode. The performance of batteries was improved as they reported for the first time a discharge potential of 1.65 V, a value close to the thermodynamic value. 12
Further, Robba A. et al. (2017) investigated the operation of an Mg–S battery using electrolytes composed of simple salts in binary mixtures of glymes. MgCl2 and Mg(TFSI)2 were dissolved in a ratio of 1:1 in a mixture of TEGDME: DOL. The study resulted in the conversion of 80% of sulfur into MgS during the first discharge. Reduction gave two plateaus while oxidation proceeded through a single plateau. However, the capacity was fading fast. 13 Manjuladevi R. et al. (2018) described a new development in the electrolyte system. They studied a magnesium ions conduction Blend Polymer electrolyte, a type of solid polymer electrolyte(SPE). These BPE films consisted of Mg(NO3)2 salts to boost conductivity and 92.5PVA:7.5PAN blend. PVA(polyvinyl alcohol) was used as a host matrix. They used PAN(polyacrylonitrile), a special conjugated polymer, to create a blend to enhance conductivity. The experiments were carried out in different concentrations of Mg(NO3)2 during synthesis to determine the effect of salt concentration on conductivity. 92.5PVA:7.5PAN:0.3 m.m.% Mg(NO3)2 composition resulted in highest electrical conductivity. 14 Supriyono., 2018 evaluated a completely new electrolyte for the Mg–S battery. This battery used 3.5%wt NaCl solution as electrolyte. He also did modeling using a simulation to obtain the optimum conditions for the usage of the battery. The discharge mechanism of the battery was studied during main and side reactions. The model was used to evaluate the maximum and minimum state of charge (SOC). 15 To further enhance the cyclicity of magnesium sulfur batteries, Wang et al.,2018 introduced a new electrolyte (PhMgCl)2−AlCl3/tetrahydrofuran-based, a nucleophilic electrolyte. The cathode was sulfur impregnated in mesoporous carbon matrix type (S@MC). The sulfur in the matrix existed as many sulfur species like S2−4 molecules and huge ringlike S8. However, the surface consisted of S8 species. The carbon matrix was chosen to improve the reaction kinetics and for adsorbing polysulphide species. Earlier, copper was used as a current collector in Mg–S batteries. 16 In the same year, Liu R. et al. (2018) demonstrated various electrolytes for Mg- S batteries. They have shown that Magnesium mono carborane (MMC) Mg(CB11H12)2 in glyme results in high anodic stability (approx 3.8 V) and an elevated Magnesium cyclicity. Magnesium hexafluoroisopropanol aluminate Mg[Al(HFIP)4]2 in ethereal solvents, the deposition of Mg is reversible with elevated conductivity (greater than 6 m Scm−1) and oxidative stability (greater than 3.5 V vs Mg/Mg2+). The thermal stability of Mg[B(HFIP)4]2·3DME is up to 150 °C, and it is also hydrolytically and air-stable, Mg[B(HFIP)4]2 is, in fact, adaptable with any cathode and anode materials which makes it an all-inclusive electrolyte for Mg batteries. The weakly cooperating nature of the [B(HFIP)4]− anion allows the effortless segregation of the salt, assists suitable Magnesium dissolution and deposition in the electrolyte. Thus the MgBhfip electrolyte can be used for cathode materials with high voltage. As stated in the common ion effect in Magnesium-Sulfur batteries with Mg[B(HFIP)4]2 electrolyte, the plateau of potential during discharge faintly rises after the first complete cycle, the voltage gap between the charging and discharging decreases. 10 Later, Wang W. et al. (2019) introduced an electrolyte of Mg(CF3SO3)2−AlCl3 dissolved in tetrahydrofuran and tetraglyme mixed solvent. DME is a potential solvent for Mg(TFSI)2 + MgCl2 electrolyte because it makes MgCl2 more soluble in the solution. Therefore MgTFSI2 + MgCl2/DME solutions result in enhanced reversible deposition of Mg. Using THF + TG mixture (in 1:1 volumetric ratio) in the form of solvent, a reduced overpotential of 0.1 V for Mg deposition and dissolution was found. The Coulombic efficiency here was found to be 89.6% at the end of the first cycle, and there is 95% stability after the third cycle, and further, the performance continues to be stable for 200 cycles. 6
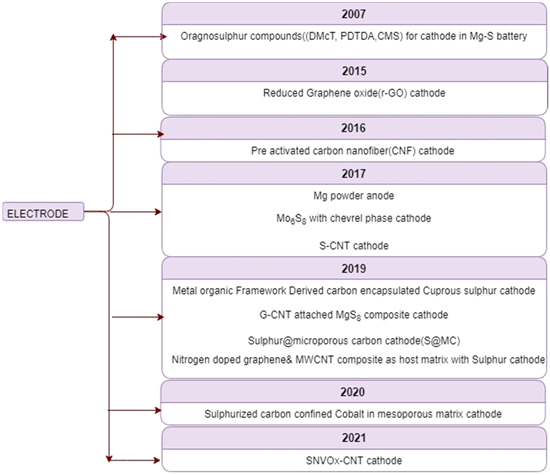
Figure 5 shows the development of carbon electrodes in the field concerning the dopping material in the current years to increase the sulfur loading of electrodes. While discussing the developments of electrodes in Mg–S batteries, an advanced class of cathode was presented by NuLi, Y. et al. (2007). These novel cathodes used organo-sulfur compounds with S–S bonds for rechargeable magnesium sulfur batteries. The battery's capacity during charge and discharge was due to the cleavage and recombination of these S–S compounds. They studied three organo-sulfur materials, namely 2,5-dimercapto-1,3,4-thiadiazole (DMcT), poly-2,20 dithiodianiline (PDTDA), and a conductive sulfur-containing material (CMS). 17 More progress occurred when Vinayan B. P. et al. (2015) developed a magnesium battery with Reduced Graphene oxide (r-GO) and Sulphur composite as cathode material. The electrolyte used consisted of a non-nucleophilic base of magnesium-bis (hexamethyldisilazide) [(HMDS)2Mg] metallated with AlCl3 dissolved in solvents of various ethers. The anode consisted of a magnesium-carbon composite. The r-GO material in the cathode was used to exploit its excellent properties like colossal surface area, elevated electronic and thermal conductivity, and increased charge mobility. 1 They reported that this kind of battery delivered a high capacity, high cyclicity, and rate capability. From the usual developments in cathode material, Sievert, B. et al. (2017) have developed and taken an incredible stride in anode material development. They reported a design of magnesium powder anode. Their studies showed an improved behavior of magnesium powder anode batteries over magnesium foil anode batteries. 18 Later, Vinayan, B.P. et al. (2019) developed a new cathode material for magnesium sulfur battery using different sulfur loadings ranging from 3–0.5 Mg–Sulfur per cm2. This cathode used nitrogen-doped hybrid nanocomposite of multiwall carbon nanotubes (MWCNTs) and graphene as a host material. They studied the generation of various polysulphide species during the charge and discharge of this battery using operando Raman spectroscopy and XPS. They synthesized Graphite oxide from Graphite, and MWCNTs were synthesized by the method of chemical vapor deposition. These materials were further mixed and treated to produce the cathodic material. 19 In further works, He et al., 2019 proposed a novel cathode using Cu on carbon nanofibers as an additive to the cathode. These composites were then mixed with sulfur to make a Cu-S cathode. They reported excellent cyclicity up to 100 cycles. Mg–S battery operations are hindered because of the powerful electrostatic attraction between magnesium cations and cathodic material. 20 To improvise the reversibility of the Mg–S battery, Sheha et al., 2019 (cathode) have proposed a new design for cathode material with V2O5(vanadium oxide) as host matrix. This can enhance the reversibility of Mg ion intercalation and extraction. 21 Since separator material in Mg–S batteries was not explored that much, therefore, Zhou et al., 2019 (separator) have demonstrated the use of novel separators for Mg–S batteries. Also, since most of the magnesium sulfur poses serious safety hazards caused due to the shortcomings of separators which can be flammable, and of shrinking nature at high temperatures, this could lead to undesirable polysulphide dissolution. Therefore, they have developed a multifunctional Janus separator of polyimide nonwovens with a copper nanowire-graphene nanosheet functional layer and an inflexible lithium lanthanum zirconium oxide-polyethylene oxide matrix. 22
Figure 5. The carbon electrode developments in different years to enhance the sulfur loading of electrodes in the Mg–S battery.
Download figure:
Standard image High-resolution imageThe reaction products formed during discharge were also able to reverse back. Moreover, the most common problem encountered in the magnesium sulfur battery operation is the polysulfide shuttling, corrosion of magnesium anode, and low cathode stability. To alleviate this issue, Sun, J. et al. (2020) introduced a design of cathode material having impregnated sulfur in carbon-confined Cobalt in a mesoporous matrix (MesoCo@C). The CoSx species played a crucial role in trapping these polysulphide species. Also, this design had the dual advantage of having Cobalt and carbon as good electron conductors. 23 Zhang et al., 2020 studied the effects of different types of binder in cathode performance. They have used sulfurized pyrolyzed polyacrylonitrile (S@pPAN) composite as cathode material. Binders studied were poly(ethylene oxide) (PEO),carboxymethyl cellulose (SCMC), sodium polyacrylate (PAAS), guar gum (GG),locust bean gum (LBG), poly(vinylidene fluoride) (PVDF), and β-cyclodextrin (β-CD). They reported that the PAAS binder showed better cyclicity and electrochemical performance compared to the standard PVDF binder. 16 To counter the sluggish kinetics of redox reactions in magnesium sulfur batteries involving Sulphur and its low electronic conductivity, Zhao et al., 2020 developed a new host for sulfur, i.e., Co3S4@MXene heterostructure. This host proved to be an effective solution as it catalyzed the conversions of polysulphide intermediates and was thus successful in improving the kinetics. Moreover, the MXene matrix allowed the diffusion of magnesium ions. 24 A new composite material as a cathodic material in Mg–S batteries was investigated by Wang P. et al. (2020). Sulfurized poly(acrylonitrile) ("SPAN") was used as a composite in their work. They have used a fluorinated electrolyte inTetraglyme solvent. A high concentration of electrolytes was preferred to reduce the dissolution of magnesium polysulfides. They studied the electrochemical results of Mg–S batteries based upon SPAN and compared it with two different types of anodes. Mg foil anode and Mg-powder anode were contrasted. Their studies showed that Mg-powder anode with SPAN gave better results as compared to Mg-foil anode. 11 Oxygen vacancies to spherical NaV6O15 cross-linked with carbon nanotubes (CNTs) (SNVOX -CNT) as a cathode material were introduced by Yang Y. et al. (2020) to achieve an excellent long-time cycle life of rechargeable magnesium batteries (RMBs). They reported that SNVOX-CNT showed a higher discharge and charge-specific capacity than CNVO and SNVO. SNVOX -CNT was able to sustain 120 mAh·g−1 after 180 cycles at 50 mA·g−1. The initial Coulombic efficiency of SNVOX—CNT reached 98%, indicating excellent reversibility. 25 The establishment of oxygen vacancies improved the diffusion kinetics of Mg2+. 26
Mg–S batteries comparison with other batteries
In Fig. 6, different reversible batteries compatible with the Mg–S battery and good charge—discharge capacities are mentioned. Lithium-ion battery (LIB) has attained great success and is currently an excellent device for energy storage for portable electronics, powering electric vehicles considering its high power and energy density and eco-friendly nature. Li has meager oxidation potential (−3.04 V) among metals, hence considered as an appropriate anode to provide high energy density for a fuel cell system. In these batteries, electrolyte typically reduces on an anode to form an (SEI)solid electrolyte interface layer that opposes more electrolyte decomposition while enabling lithium-ion movement. Also, the Earth's crust has an uneven and lesser distribution of lithium metal resources. However, currently used lithium-ion batteries cannot meet the increasing requirements for higher energy density, low cost, and safety. The need for Li-ion batteries is expected to increase in the future, and hence it raises concerns about its demand fulfillment due to lack of element sources. Lithium-sulfur batteries have higher potential, seeing as it is cheap and less toxic nature of sulfur, and significantly higher theoretical capacities of the sulfur cathode (3459 mAh ml−1, 1672 mAh g−1) and lithium anode (2046 mAh ml−1, 3861 mAh g−1). 22 However, dendrite formation and weakened coulombic efficiency during the repeated deposition-dissolution limit the growth of these batteries. In addition to this, its price and lack of resource availability of Li metal reduced its Large scale applications. In Li-S batteries, current collector Cu and powdered Cu were used to increase the electrochemical property of sulfur cathodes in lithium-sulfur batteries by converting sulfur to electrochemically active Cu2S. In these batteries, there is no such complex multielectron solid-liquid-solid conversion as in the Mg–S battery. Usage of separators in both Li-S batteries and Mg–S batteries improves battery-safe operation and electrochemical properties of batteries. When the polysulfides are generated, the shuttling phenomena occur, resulting in loss of capacity, corrosive nature of anode, and coulombic efficiency decay. Researchers have also focussed on several new technologies which have emerged, such as magnesium-ion batteries (MIBs), aluminum-ion batteries (AIBs), potassium-ion batteries (KIBs), and sulfur-ion batteries (SIBs). 27 Benefitting from sulfur's low redox potential, it can be easily combined with metals such as Na, K, and more, increasing the cell voltage of the particular cells. The energy density gets maximized in this case. The formation of sulfides from sulfur leads to significant volume change in the cells. There is an expansion in the volume in the range of 80% (S to Li2S) to 300% (S to K2S), indicating more space is required, and also solid electrodes can crack. There is more dendrite growth as well as electrolyte decomposition in the cells. Table I given below shows how advantageous the various properties of Mg are compared to different metallic anodes. The Na-S battery is the currently developed high-temperature battery that operates at near 300 C, which is also based on liquid electrodes such as molten sulfur or polysulfides and molten sodium, which are set apart throughout the solid electrolyte. Many other membranes are used to prevent the polysulfides shuttle phenomena from the electrodes. Polymer-based membrane material Nafion suppresses the shuttle effect in Li-S and Na-S electrochemistry. Recent observations declared that there is no complete suppression. Finer overall cell performance and cycle stability have been displayed when kept at room temperature in Na-S batteries. On the contrary to Mg electrolyte, potassium electrolyte solutions are prepared with traditional salt in solvent methods. Potassium metal electrodes provide 685.5 mAhg, which is the lowest among the metals. The theoretical voltage of K–S cell (1.88 V) is close to Na-S cell but is not very high to Li or Na and fulfills the requirements. Although, the lower melting point of K for liquid electrode understanding can be beneficial. 28
Figure 6. Competing reversible metal batteries/power sources to Mg–S battery.
Download figure:
Standard image High-resolution imageTable I. Comparison of various properties of magnesium metal with different metallic anodes. 27
| Parameters | Li | Na | K | Mg | Ca | Zn | References |
|---|---|---|---|---|---|---|---|
| Ionic radius (Å) | 0.76 | 1.02 | 1.38 | 0.72 | 0.99 | 0.75 | 27 |
| Atomic mass (gmol−1) | 6.94 | 22.99 | 39.10 | 24.30 | 40.07 | 65.38 | 27 |
| Voltage (V) | −3.05 | −2.71 | −2.92 | −2.35 | −2.84 | −0.78 | 27 |
| Abundance in earth's crust (wt%) | 0.0022 | 2.56 | 1.5 | 1.35 | 2.94 | 0.0078 | 27 |
| Capacity (mAhg−1) | 3861 | 1165 | 685 | 2205 | 1337 | 820 | 27 |
| Cost (US$ ton−1) | 165000 | 200 | 1000 | 4600 | 110000 | 2570 | 27 |
Rechargeable Mg-ion batteries have an excellent capability to supersede Li-ion batteries because of the properties like the absence of formation of dendrites during cycling, having excellent energy density, and being less expensive. Table II gives insights into the high performance and operation when pure sulfur is used as a cathode for lithium and Magnesium sulfur batteries. Magnesium metal has shown more superficial processing features, lesser reactivity toward moisture or air, and lesser dendrite formation than lithium gaining from the strongly bonded magnesium(Mg) atoms along with better ion mobility. These are significant for the current developing technology because lower costs, high energy density, and safety make metallic Mg a potential anode in practical batteries. Also, the large size of ion and slow diffusion capacity of bi-valent Mg ion(Mg2+) slows the kinetics of the reaction to advance cathode reaction kinetics to gain fast Mg conduction and diffusion. However, the large size also leads to high polarisation, which results in the diffusion of Mg2+ ions in the host materials.
Table II. Performance-based comparison of metal-sulfur batteries.
| Parameters | Cathode | Temperature (℃) | Current density | Cycle number | Capacity (mAh g−1) | References |
|---|---|---|---|---|---|---|
| Mg–S | Pure sulfur | 80 | 0.5 | 50 | 817.8 | 29 |
| Li-S | Pure sulfur | 50 | 100 | 25 | 915.3 | 29 |
In Table III, a comparison based on various parameters is drawn between the RMBs and other metal sulfur batteries. It demonstrates the high performance of RMBs than the metal-sulfur battery as a promising safe and better design with the durable operation of the battery for future generations.
Table III. A comparison of various parameters of the RMBs.
| Batter y | Coulombi c efficiency | Initial discharge capacity | Cycles | Electrolyte | Electrode | References |
|---|---|---|---|---|---|---|
| Mg–S | 99.8% | 830 mAhg−1 at 0.1 C | 400 | BCM | MesoCo@C cathode | 23 |
| Li-ion | 270 mAh−1 | 100 | LiPF6 2FEC/DMC/F- EPE | Li1.2Mn0.56Co0.08Ni0.16 O2 | 30 | |
| Mg ion | >90% | 118 mAh g−1 | 50 | dual-salt electrolyte of Mg-APC and Na(CB11H12) | NaCrO2 cathode | 31 |
| Li-S | 95% | 977 mAh g−1 at 0.1 C | 60 | 1 MLiTFSI + 0.1 M LiNO3 in DME solvent | Carbon Foam@CNTs/MgO-S | 8 |
| Na-S | 79.1% | 1541 mAhg−1 at 0.1 C | 200 | 2 M NaTFSI in PC: FEC with InI3 | S@MPCF | 32 |
| K-S | ∼97% | 512.7 mAhg−1 | 50 | 1 M KClO4 in TEGDME | Polyacrylonitrile (cathode) coated with CMK-3/Sulfur and K metal (anode) | 33 |
Cathode materials for magnesium based batteries and their practical applications
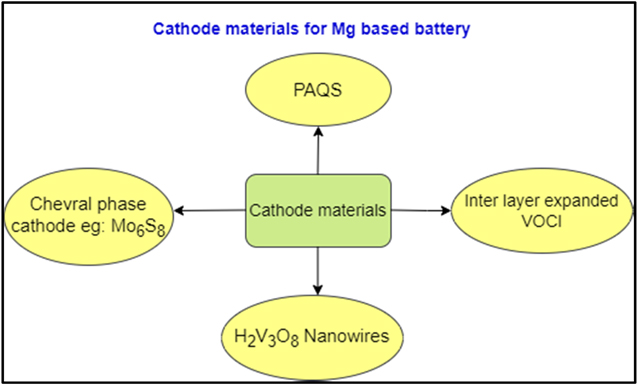
We know that Mg ion batteries have become very popular due to their ability of storing large amount of energy in energy storage materials, which are used in huge mobile and immobile appliances. Recently vanadium oxychloride has been utilised as cathode material in Mg based battery. But unfortunately, the battery delivered a very capacity of 45 mA h g−1 in the first cycle. For improving the results, the interlayer of VOCl has to be expanded for the easy insertion of Mg ions into the structure of electrode material. Therefore, the VOCl cathode was cycled with Li. As a result of which, the spacing between the layers of cathode increased and thus higher capacities were obtained. 170 mA h g−1 capacity was obtained after the successful treatment. 34 Vanadium oxides have always been an important option as electrode materials. This may be due to their cheap cost and abundance. Furthermore, H2V3O8 nanowires has been evaluated as cathode material. But here, the spacing between the layers is greater in V3O7 structure as they are connected by H-bonds. The battery displayed a very high specific capacity of 305. 4 mAh g−1. The battery retained 261.2 m Ah g−1 even at the end of 20 cycles. 35 Fig. 7 represents various other types of cathode materials have been studied for rechargeable Mg based batteries. Another type of cathode material is the Chevral phase, they consist of Mo6S8, Mo6Se8, Mo6Te8. They also work as intercalation materials accommodating Mg ions. These materials are crystalline in nature, with S8 anions occupy the corners and Mo6 atoms occupy the face side. However, a battery using this cathode material gives low capacity on discharge. And for maximum it has to be operated at a higher temperature. 36 Polymer based materials have also been evaluated as cathode materials. One of them is PAQS(poly(antraquinoyl) sulfide). It was developed to increase the cycling efficiency of battery. This battery gave an initial discharge capacity of 100 mAh g−1. The cycling efficiency was also found to be moderate, as after 80 cycles the capacity reduced to 60 mAh g−1 from 100 mAh g−1. 37
Figure 7. Cathodes that have been developed in the recent times for Mg based battery have been shown and discussed.
Download figure:
Standard image High-resolution imageElectrodes in Mg–S Battery
Electrodes used in various research works
Herein, we report the development of different electrodes that can be used in Mg–S batteries. Cheng Y. and his coworkers in 2012 reported a Li+ intercalation cathode (Mo6S8) and Mg anode. This battery delivered an excellent performance rate (about 83% retention of capacity at 15 C). These hybrid batteries show outstanding rate capability, reliability, and superior safety with suitable discharge voltages. This battery showed low capacity fade (∼5%) after 3000 cycles, and the Mg metal surface was also clean and smooth with no dendritic structure. These Chevrel phase compounds display good Mg2+ ions intercalation characteristics. However, the diffusion of Mg2+ ions is relatively slow, and the rate capability is also constrained at room temperature. 38
Shunsuke Yagi et al. 2013 used a glassy carbon electrode and coating the same on Ti and stainless steel electrodes with a Grignard reagent-based electrolyte. 39
Huiea et al., 2014 presented that magnesiated Prussian blue or Platinum is used as a counter electrode and water considerably plays a crucial role in the electrolyte and cathode crystal to help determine the characteristics of aqueous Prussian blue Mg-based battery. As observed in hydrated V2O5, water present in interstitial sites within Prussian blue structure helps disturb Mg2+ ions as it is very well known that the use of water in Mg batteries causes the magnesium oxides to accumulate on the surface of the anode because of the reaction between Mg and water and it restricts the usage of water in Mg batteries. Magnesium oxide formed on the surface leads to power and current losses as they are unstable and inhibits ion diffusion. Various cathodes materials for Mg and Mg-ion batteries are molybdenum oxide, molybdenum sulfide, Chevrel phases, vanadium oxide, manganese oxide, Prussian blue, and transition metal silicates. Mg ion transfer properties are enhanced by using layered vanadium oxides, which exhibit high capacity, high surface area, and enhance operating voltage and formation of tiny crystallites with acceptable water content. Manganese oxides provide a range of structural forms with varying electrochemistry. The layered spinel structured materials (birnessite) presented better cycling than (hollandite), with a tunneled structure. Irreversible structure changes are induced by insertion and removal of divalent charges through charge trapping exhibited by orthorhombic structured molybdenum oxides, which thin films could address. One of the most studied cathode materials for Mg batteries, Molybdenum sulfides exhibit less structural degradation upon cycling because of less structural rigidity than oxides. Although slow diffusion rate and magnesium ion charge trapping are still an issue, high-temperature cycling for improving diffusion rate and nanomaterials' usage can better perform. Molten salt and sol-gel synthesis methods are used to prepare silica with iron, cobalt, or manganese, which is a key to generating viable electrochemical performance in Mg batteries. The advantage of involving molecularly based aqueous systems and active cathode materials having a structure analogous to Prussian blue (nickel and copper hexacyanoferrate) is that it paves pathways for Mg2+ ions mobility. 40
Shuojian Su et al. 2015 introduced an Mg–S battery with a titanium dioxide cathode containing Mg anode coupled with magnesium borohydride/tetraglyme electrolyte. Commercially available TiO2 was used without further treatment and investigation of TiO2 to be used in batteries. The capacities at various current rates from 0.1 to 2 C were measured for evaluating the rate performance of TiO2. It was noted that TiO2 exhibited almost the same theoretical capacity and initial discharge capacity. The Mg–S batteries showed enhanced rate capability and capacity. 41 NaWu et al., 2015 exhibited an improved electrochemical performance of Li4Ti5O12 electrode in an Mg battery system. This was done by and Li+ and Mg2+ co-insertion into large-sized LTO electrodes. It showed excellent energy storage capability of different large-sized electrode materials, effective in rechargeable Mg batteries. 42 Bucur et al. 2015 mentioned that some cathode substances which showed better performance in Li-ion batteries did not respond the same way in Mg batteries showing poor reversibility; this suggested that the variety of compatible materials can be entirely different for Magnesium batteries than in Lithium batteries. The effect of water on the cathode's overall performance can be used to enhance it if the electrolyte stability and Mg electrode reactions can be minimized. Electron delocalization is preferred over transition metal oxidation/reduction to improve charge stability in insertion cathodes. An electrolyte that can be catalyzed through highly electropositive cathodes can form blockading films through their oxidation, which is considered one of the most significant issues. Ether solvents exhibit good reductive stability, making them suitable for most successful Mg electrolytes, whereas their oxidative stability is not as good as that of carbonate solvents used in Li-ion battery electrolytes. 43 Itaoka et al.,2015 showed that sulfur-containing composite electrodes showed a faster electrochemical reaction rate when compared between triglyme electrolyte and acetonitrile electrolyte. The cyclic ether unit has a better cycling behavior of the cell with the sulfur-containing composite electrode than the linear ether unit. The reason behind that may be the formation of the paths for ion conduction in the cathode by the cyclic ether units and Mg ions. An increase in the internal resistance can be prevented by these paths. 44
Bonnick P. et al. (2016) suggested layered TiS2 as a favorable intercalation material for positive electrodes. TiS2 electrode particles are micrometer-sized. This layered TiS2 is prepared by grounding titanium powder with 10% excess sulfur. For the Mg'sentire cell, this provides a 115 mAh g−1 capacity. By cycling at high temperatures, the excessive migration barrier to magnesium diffusion can be reduced. With the partially magnesia TiS2 formed on the first cycle, reversible Mg2+ de/intercalation can be attained. 5 Vinayan et al.,2016 presented the Mg–S mobile in which sulfur diffused reduced graphene oxide because cathode gives excellent cyclability and a reasonable charge functionality because of reduced graphene oxide's unique purposeful and morphological properties. An Mg/S battery with exact cyclability the usage of a graphene-based sulfur composite electrode and a non-nucleophilic electrolyte. Reduced graphene oxide represents a buffer layer, houses the quantity modifications on top of electrochemical cycling among sulfur and magnesium sulfide, and gives accurate electronic conductivity and an excessive surface area for the dispersal of active material. 45
Sievert, B. et al. (2017) have reported a novel Mg–S battery design using Mg powder anode. They synthesized the metal anode using magnesium powder(99.5%) and graphite powder. They prepared two different powder anodes, namely PALP(powder anode at low pressure) and PAHP(powder anode at high pressure), depending on the pressure they applied to this powder while pressing it in a hydraulic press. They compared these electrodes with magnesium foil anodes. Their research has utilized the 50 S and 70 S cathodes, where 50 and 70 represent the mass fraction of sulfur in the cathode. They reported that Mg-PALP cathode in 50 S and 70 S delivers a very high capacity of about 600 Ah/Kgsulfur in the first cycle. The capacity fades quickly. Moreover, an insufficient capacity was obtained after ten cycles.
Their experiments reported that the PALP magnesium anode absorbs the electrolyte while the PAHP anode does not. PAHP anode is more similar to foil anode due to its dense structure. PALP anode showed more clearer voltage plateaus in both charge and discharge as compared to PAHP anode. PAHP anode produced a single plateau, and the capacity retention was not good at least up to ten cycles. The charge/discharge profiles of PAHP anode and foil anode were similar. To conclude, powder anodes show better results than foil anodes. 18 Tian, H. et al. (2017) studied the use of Iodine as a cathode material. Here, I2 can be reduced to I3 −. Its reversible redox reaction involved I3 − to I−. The battery could deliver a capacity of 180 mAh g−1, a voltage of 2 V. The capacity retention was about 95% at 0.5 C rate even after 120 cycles. 46 Minella et al., 2017 showed that the original, non-increased VOCl confirmed no magnesium intercalation/deintercalation. After the primary discharge at 298 K, the specific potential was enhanced by an aspect of 4 to 170 mAhg−1 because of Li pre-treatment. During the first recharge in Mg-cell, the Li present in the VOCl structure goes back and forth from cathode to electrolyte, following the concept of hybrid batteries. Intercalation of magnesium is caused after this, and it exists over the Li intercalation, which is confirmed by battery testing. Easy and quick in situ methods have been presented to customize layered nanostructures in non-aqueous electrolytes and the adaptability of VOCl for electrode applications. 47 Karger et al., 2017 showed that nucleophilic, organomagnesium compounds could damage the electrophilic cathodes, which include sulfur; otherwise, they are, ironically, pivotal in synthetic organic chemistry. The nucleophilic attack at the sulfur cathode was mitigated utilizing crystallizing the electrochemically energetic species from a 3:1 mixture of HMDSMgCl: AlCl3. The wider voltage window and improved coulombic performance, and electrochemical blessings are carried out through crystallization. XPS analysis supplied us with a method to reveal the electrochemical conversion of the cathode while it was charged and discharged. 24
Du et al., 2017 effectively synthesized a singular SGDY cathode for Li–S and Mg–S batteries, displaying first-rate electrochemical performances including advanced rate capability, potential solid retention, high coulombic efficiency, and massive ability, except for the first cycle. Electrolytes like the commonly used Grignardreagents-based electrolyte and commercially available carbonate electrolytes are all stable with this cathode. The richness and flexibility of the graphyne family confirmed the high and strong ability of assembled Mg–S battery as much as 36 cycles, which is advanced to previous reviews, this is because of its promising ability to enhance the content material of sulfur through tuning graphyne structure, thus presenting a considerable potential towards high-overall performance and low-cost sulfur-based batteries. 48
Manjuladevi R. et al. (2018) have reported a Blend polymer electrolyte film capable of conducting magnesium ions. This Mg–S battery has used Mg anode, BPE film as an electrolyte, and MnO2 and Graphite composite cathode. Graphite was used in this to make the cathode electronic conductor. Following are the reactions occurring in the battery-
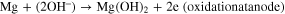
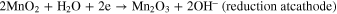
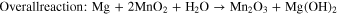
The source of hydroxyl ions is from the structure of PVA, which is the host in BPE films. Also, due to the porous nature of the film, there is some moisture present called occluded moisture. 14 Kong et al., 2018 proposed that a cogent design of S hosts as the cathode is favored to accomplish the high overall performance of Mg–S batteries. Consequently, for the durability of Mg–S batteries, a unique design of conductive hosts is required to undergo vigorous chemical interactions with Mg-PS that were based entirely on interfacial phenomena instead of spatial confinement to diminish the back and forth effect and enhance the S utilization through its high electric conductivity. Stable cyclic performance in Mg–S batteries is achieved by using porous nanostructured materials, which provide enough interfaces and the uncovered surfaces provide an opportunity to enhance to anchor Mg-PS. Practical techniques to shield the metal surface are using high capacity alloy anode substances and a suitable protecting and conductive synthetic interphase on Mg anode. Crucial mechanistic understandings of running Mg–S batteries also need attention other than the requirement of substantial breakthroughs in the sulfur cathode, magnesium anode, and electrolyte. 49 Wu et al., 2018 reported that, for Mg secondary batteries, CuS nanoparticles could be used because of their extreme performance as a cathode. The cathode shows incredible cycling stability (over 350 cycles), high ability (one hundred seventy-five ma hg−1), and excellent rate capability. Small-sized particles favorable for the conversion response are formed due to the higher conductivity of Copper Sulfide and slow stimulation of the electrode. A decrease in sulfur content was observed for CuS while investigating the mechanism of transformation of MgS to CuS, and reversible conversion response paramountly came about between Cu and Cu2S. 50
Peng He et al., 2019 works have suggested that using Cu current collector at the cathode improves the cyclability. Here the cathode was made with both h-Cu@CNF and Cu@CNF and magnesium disk anode. The sulfur loading of these cathodes was in the range of 0.06−0.12 mg cm−2. 20 Raphael Richter et al., 2020 used sulfur composite electrodes were prepared by the widely used melt infiltration method and a magnesium metal foil with 18 mm diameter as an anode. The kinetics of the side reactions on the anode surface has a high impact and can be responsible for the rapid self-discharge in Mg–S cells. 29 Meng et al., 2019 proposed that Mg3Bi2 was turned in as an alloy-type negative electrode due to how bismuth behaved in Mg batteries. However, using Mg2Sn or Mg2Pb anodes, higher theoretical electricity densities could be expected. 51
Shuxin Zhang et al., 2020 reported using S@pPAN cathodes with Sodium Polyacrylate as a great aqueous binder for Magnesium−Sulfur batteries. The PAAS-based S@pPAN electrode exhibits good high rate capability and cycling stability due to its amorphous structure and an overlapping 3D network formed after the cross-linking upon cycling. It showed improved electrochemical performance of electrochemical batteries. 52
Divyamahalakshmi Muthuraj et al., 2021 introduced a magnesium polysulfide (MgSx) catholyte as a liquid-phase active material and Mg anode to conquer the sluggish redox kinetics of Mg/S batteries. 53 Talian et al., 2021 showed that the impedance spectroscopy outcomes on porous carbon electrodes also offer data on the bottle-neck procedures in the course of their operation, that is, the diffusion of polysulfide species from the majority of the electrolyte in the separator in the direction of the electrode surface.5 l et al., 2020 reported that KB/S is amalgamated by the melt diffusion method. The cathode electrodes were formulated by casting KB/S on the current collector, which was beneficial to boost conversion kinetics of the sulfur cathode. The stoichiometric Ketjenblack carbon (KB) and sulfur were soaked in CS2 and agitated for 12 hr to volatilize CS2. The KB/S composites were acquired after the mixtures were pulverized and heated at 155 °C under Argon atmosphere for 12 h. 54
Carbon electrodes in Mg–S batteries
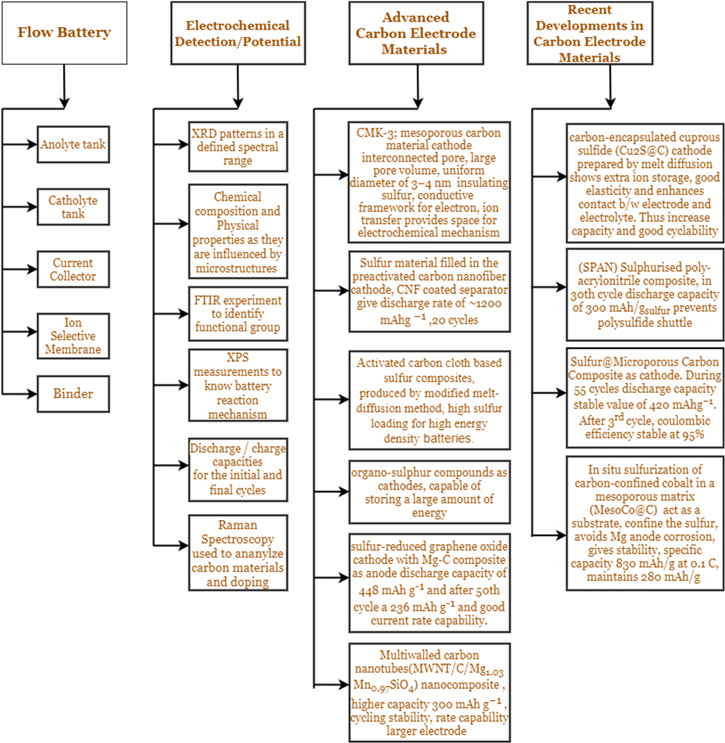
Carbon is known as a highly conductive agent as it widely helps improve the material's conductivity. Carbon has proven to be a simplified and efficient way to ensure adequate access to electrons. MWCNTs are also good conductivity agents to ensure mechanical integrity and smooth mobility of the Mg2+ ions and electrons. Graphene, an allotrope of carbon, is another conductive agent because of its excellent thermal, electrical, and mechanical properties, improving the low electronic conductivity of materials. Carbon is used as a host matrix in most Mg–S batteries because there are always some large overpotentials between charging and discharging processes in most Mg–S batteries. Capacity fading is fast. There is also a loss of active material. These problems are enhanced when sulfur loading in cathodes is high for more than >1 mgsulfur cm−2. These problems correspond to the formation and shuttling of soluble polysulphide species between cathode and anode during battery operation. Sometimes, the inferior performance is also due to the slow reaction kinetics of magnesium ions. These polysulphide species at the anode increase anodic impedance and causes a considerable lag between charge and discharge values. Hence, to avoid the problems mentioned above, the trapping or confinement of polysulphide species is essential. Therefore, cathode materials with a polar conductive host matrix with a large surface and high affinity for sulfur species can help bind them by adsorbing them. 19 Also, they enable the easy transfer of electrons and ions through them during reduction and oxidation. From Fig. 8, we can look at the various developments in the carbon electrode as discussed further in the section and different carbon electrodes used in various studies and research work.
Figure 8. Carbon electrodes can be made up of several carbon materials in which carbon is present directly or modified.
Download figure:
Standard image High-resolution imageIn Table IV, we gathered different materials doped while preparing the carbon electrode and the latest developments in the fabrication of carbon-based electrode composites to improve the overall electrochemical performance of the battery. Wang W. et al. 2020 proposed a carbon-encapsulated cuprous sulfide(Cu2S@C) cathode of this Metal-Organic Framework- derived cuprous sulfide prepared by a melt-diffusion method. In this, through ball-milling, sublimed sulfur and Cu-MOF were mixed after that carbonization process was done. The significant amount of carbon in composite posses good elasticity. The surface area of Cu2S@C composite carbon enhances the contact of electrode and electrolyte by this polarization decrease, and capacity improves. Extra ions stored at the Cu2S@C surface also increase the capacity. This cathode shows better cycle stability as compare to Cu2S cathode due to MOF-derived carbon frameworks. A slower current decay, more negligible voltage polarization, and l larger peak currents can be seen in Cu2S@C∣Mg cell. 23 Wang P. et al. 2020, proposed a Sulfurised poly(acrylonitrile)(SPAN)composite.SPAN is synthesized by reacting PAN, and excess S8 in a nitrogen atmosphere and electrodes are prepared by coating poly(vinylidene difluoride):SPAN: carbon black in N -methyl-2-pyrrolidone (NMP) on an Al/C foil. By pressing Mg powder with a hydraulic press, the anode is made. In this cathode, sulfur is chemically bound to the polymeric backbone. Poly(sulfide) shuttle-free cycling discharge/charge rates of 8 C for more than 1100 cycles with less than 10% loss is obtained. In the 30th cycle, a discharge capacity of ca. 300 mAh/gsulfur is reached. Mg–SPAN cells are one the best Mg–S cells if the electrolyte contains magnesium conductive salt. 55 Manthiram A. et al. 2016 studied a sulfur material filled inside preactivated carbon nanofiber (CNF) electrodes, and the separator is also coated with CNF; this separator acts like a polysulfide trapper. After cycling, homogeneous distribution of sulfur is achieved. CNF coating is porous so that it can absorb diffused active cathode materials. Contrarily, CNF coating is electronically conductive, due to which it will act as a current collector because of this cyclability, and sulfur utilization is improved. A discharge capacity of ∼1200 mAhg−1 is obtained and lasts up to 20 cycles. 56
Table IV. Materials doped in carbon electrode & its effect on the overall battery's working & efficiency.
| Cathode | Electrolyte | Sulfur loading | Initial discharge capacity (mAhg−1) | Overpotential (V) | Coulombic efficiency | References |
|---|---|---|---|---|---|---|
| MesoCo@ C−S | [(HMDS)2Mg]+AlCl3 + MgCl2 in tetraglyme solvent | ∼40wt% | 830 mAhg−1 at 0.1 C | >0.5 | 90% | 23 |
| S/C | BCM | 85wt% | 1081 | 0.4 | 99.8% | 57 |
| S/NC | Mg[B(hfip)4]2. 3DME | 20 wt% | 1384 mAh g−1 | No data available | No data available | 19 |
| S/CMK400P EG | Mg-TEGIL | 55wt % | 800 mAh g−1 | No data available | No data available | 12 |
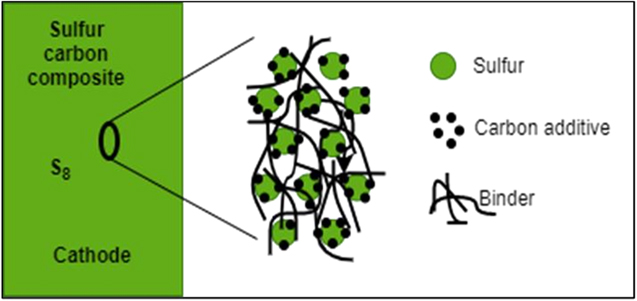
Figure 9 explains the cathode structure when carbon microparticles surround sulfur particles as binder material providing stability to the S-C composite. Nuli Y. et al. 2019 prepared Sulfur@Microporous Carbon (S@MC) Composite as a cathode. A composite containing 55 wt % sulfur content was prepared by the melt diffusion method by ball milling a mixture of sublimed sulfur and microporous carbon in a 1:4 ratio. Coin cell was constructed using S@MC as cathode,Mg ribbon anode and Mg(CF3SO3)2 + AlCl3 + MgCl2 + anthracene/THF + TG as electrolyte. During 55 cycles, discharge capacity keeps a stable value of 420 mAhg−1.During 200 cycles, cell performance was stable, and after the third cycle, coulombic efficiency was 95%. 6 Zhao-Karger, Z.et al. (2014) have demonstrated a magnesium sulfur battery using [(HMDS)2 Mg] based non-nucleophilic electrolyte and CMK-3 based cathodes. CMK-3 material is a mesoporous carbon material. This material has a huge pore volume which provides a conductive framework for electron and ion transfer. The cathode is fabricated by filling liquid sulfur into these pore spaces. When the heating is done at 160- degree Celsius, the cathode is called S/CMK160, and when at 400 degrees celsius, S/CMK400. They used PEG as a coating material for these cathodes. Moreover, two binders, PVDF and CMC, were compared for their application in these cathodes. The cells using the CMC binder displayed a relatively lower initial discharge capacity. However, the charge- discharge profiles were similar. Cells using PVDF binder in TEGIL electrolyte delivered the highest capacity of 800 mAh g−1 in the first cycle. However, the value dropped steeply to 350 mAH g−1 in the second cycle. However, cells using PVD or CMC binder in the same electrolyte solutions could maintain about 260 mAh g−1 capacity even after 20 cycles. 12 Wang W et al. (2018) prepared a sulfur@ microporous carbon (S@MC) composite with an excellent S loading (64.7 weight %). This provides stabilization of S2−4 small molecules in microporous carbon, and chemical bonding of copper to large molecules such as S8 with non-nucleophilic electrolytes also provides a productive approach to advance the electrochemical functioning of Mg−S batteries. 58
Figure 9. The structure of carbon cathode sulfur particles surrounded by the carbon microparticles binder material, which gives the sulfur carbon composite stability.
Download figure:
Standard image High-resolution imageActivated carbon cloth (ACC) is an anchor material in magnesium-sulfur batteries with permeable carbon materials. Here also, the S-ACC composite is infused with sulfur to make cathodes. This cathode can also accommodate a higher amount of sulfur for high-energy- density batteries. Gao, T. et al. (2018), in their study of the kinetics and thermodynamics of this cathode, showed that as the amount of S per carbon increases, the capacity decreases. During the first stage of reduction involving S8 to MgS8 and further reduction to MgS2, the kinetics is independent of the S/C ratio, but during the last step(MgS2 to MgS), there is a termination of discharge due to large overpotentials, and this can be more pronounced at high S/C ratio. They reported that the cell had an initial cut-off voltage of 1.4 V and coulombic efficiency of 100%. When the cut-off voltage is 0.5 V, the coulombic efficiency is 82%. 59 Dai W. et al.,2018 used activated carbon cloth (ACC) based sulfur composites. This was produced by an improved melt-diffusion method with round about 1 mgcm−2 sulfur loading. Swagelok-type cells with MgBhfip/DME electrolyte, Mg foil anode, and ACCS cathode give a first discharge capacity of 1000 mAh g−1, and at the end of 20 cycles 660 mAhg−1. 10
Sun, J. et al. (2020) introduced an in situ sulfurization of carbon confined Cobalt in a mesoporous matrix (MesoCo@C), which could alleviate the Mg anode corrosion. This in situ sulfurization system takes place by inserting melted sulfur inside the mesoporous carbon- confined cobalt matrix. Due to the vast amount of surface energy of nanosized Co particles, their surfaces get oxidized by the hot sulfur melt forming CoSx bonds at the surface. The CoSx species can control the polysulfide shuttle. In-situ sulfurization technique to supply carbon-confined sulfurized Cobalt in a mesoporous matrix (MesoCo@C−S).MesoCo@C−S cathode has a very smooth surface after cycling. Mesoporous carbon structure acts as a substrate material for the confinement of the sulfur, and Cobalt in bulk has conductive properties to allow transfer of electrons; The MesoCo@C−S cathode has 40% sulfur loading and has good electrochemical performance with a specific capacity of 830 mAh g−1 at 0.1 C and maintains 280 mAh g−1 capacity after 400 cycles. The battery uses Mg-foil anode and MMAC-DME as electrolytes. The coulombic efficiency was also up to 70%-80% during the first ten cycles. The charge(2.2−2.5 and 2.5−3 V) and discharge(1.8−1.5 and 1.5−1.2 V) profiles showed two plateaus. Compared to the CB-S cathode, the MesoCo@C−S cathode gave 780 and 380 mAh g−1 at 0.2 and 0.4 C, while the CB-S cathode could give 200 and 80 mAh g−1 of capacity. 23
Chen et al. prepared the multiwalled carbon nanotubes(MWNT/C/Mg1.03Mn0.97SiO4 nanocomposite) by the one-step CVD method. The hierarchical structure of the MWNT and coated carbon layer provided a larger contact surface of electrolyte or electrode. When used against Mg anode, it exhibits a higher capacity of 300 mAhg−1, better rate capability, and cycling stability than the pure Mg1.03Mn0.97SiO4 nanoparticles and carbon-coated composite. 60
Zhuang Y. et al. (2021) developed oxygen vacancies to spherical NaV6O15 cross-linked with carbon nanotubes (CNTs) (SNVOX -CNT) as a cathode material. The storage capacity of Mg2+was enhanced due to the introduction of metal ions, i.e., Na + in layered transition metal oxide, i.e., V2O5. Insertion of Mg2 + inside the cathode is responsible for the electrochemical performance of the cathode. By the Oxygen vacancies, diffusion can be improved. SNVOX - CNT maintains 120 mAh·g−1 after 180 cycles at 50 mA·g−1.SNVOX-CNT shows 98% initial Coulombic efficiency. This electrode can maintain its morphology after 50 cyclings. Therefore, Vinayan, B.P. et al. (2019) developed a new cathode design with a host matrix consisting of nitrogen-doped hybrid nanocomposite of multiwall carbon nanotubes (MWCNTs) graphene. The cathode used different sulfur loadings ranging from 3 to 0.5 mgsulfur cm−2. For 3 mgsulfur cm−2 loading in the cathode, the cell displayed a capacity of 431 mAh g−1 during 1st, 406 mAh g−1 during 2nd, and 228 mAh g−1 during 50th cycles. The capacity retention is about 94.2% and 53% during the second and 50th cycles. The capacity fade may be attributed to the high sulfur loading, which can augment polysulfide dissolution, and also, there is less utilization of sulfur in the cathode. When loading is decreased to 0.5 mgsulfur cm−2, the capacity during the first cycle increases to 1384 mAhg−1. 19
Nuli, Y. et al. (2007) have proposed a new type of cathodes using organo-sulfur compounds. These electrodes consist of S–S bonds, and the capacity generated during charge and discharge is because of the breaking and recombination of these bonds. They have suggested that the redox reaction between (-SH) thiol and (S–S) disulfide compounds can store a large amount of energy. The theoretical energy of these compounds is more than many polymer composites and intercalation compounds. They have used PDTDA(Poly-2,20 -dithiodianiline ), DMcT( 2,5-dimercapto-1,3,4-thiadiazole) and PAn(polyaniline ) composite and CMS(conductive sulfur-containing material). For DMcT and PAn composite, the capacity of first discharge was only 16.8 mAh g−1. This value increases up to 29.2 mAh g−1 during the tenth discharge cycle. They also observed two voltage plateaus at 1.4, and 0.9 V. For PDTDA cathode, the battery discharge capacity was reported to be 78 mAh g−1 in the second cycle. This cell exhibited low degradation of capacity even after 30 cycles. The reason may be the structure of PDTDA which enables intramolecular catalysis. CMS contains the main chain to provide electric conductivity, and the sidechain contains disulfide bonds. Since the performance of only CSM cathode was inferior, they have used CSM-PAn composite. It gave a discharge capacity of 117.3 mAh g−1 during the first, 93.0 during the second, and 72.7 mAh g−1 during the twenty-second cycle. 17
Diemant T. et al. 2016 has used a sulfur-reduced graphene oxide cathode made by the graphite oxide (GO) thermal exfoliation at 400 °C in an argon gas atmosphere. Mg-carbon composite is used as an anode prepared by together ball milling of conductive carbon black and Mg powder in 20:80 ratio. Graphene (G) has high charge mobility, high mechanical strength, huge surface area, good chemical stability, and outstanding thermal and electronic conductivity. Due to these properties, G is preferred for energy storage applications. The presence of an oxygen functional group over the rGO surface helps increase the bond formation with sulfur, which helps improve the capacity. This cell offers a good current rate capability and reversible discharge capacity of 448 mAh g−1 and after the 50th cycle 236 mAh g−1. Wang P. et al.2019 prepared the graphene-wrapped V2O5 microparticles by the solvothermal reaction. While used as cathode in Mg(AlCl2BuEt)2/THF electrolyte against the Mg foil counter and electrodes, the assembled composite can provide a high capacity of 178 mAh g−1 in the initial discharge. V2O5 cathode with the presence of graphene demonstrates excellent, more extraordinary performance as a complete Mg cell cathode. 55
Material characterization of carbon electrodes in-comparison with other electrodes in used Mg–S battery
Several types of electrode materials are used in Mg–S batteries, as discussed above, because of their different microstructure. The material's microstructure highly influences the physical properties like conductivity, hardness porosity, absorption, corrosion resistance, etc. An electrode mainly consists of a binder, current collector, active material, and doping material. From Fig. 10, we understand the microstructural arrangement inside an electrode and a current collector and binder, strengthening the bond between carbon and active material. The microstructure of these components has a high impact on the ionic conductivity, Large specific capacity, cyclic stability, and coulombic efficiency because the material structure decides the electrode's reactivity with electrolyte and microstructures vary considerably with the processing or synthesis conditions and methods. So understanding microstructure is very important to find the cell performance. The crystal structure is an inherent property of a material, but the crystal parameters can be slightly modified by introducing dopants. The introduction of Na+ to V2O5 resulted in enhanced cyclic stability 26 and enhanced the storage capacity of Mg2+. Scientists performed different diffraction and scanning techniques for getting information about the electrode materials' microstructure. In Mg–S batteries, the anode is mainly made up of magnesium metal only. In order to improve its physical properties, different forms of this material like Mg foil, Mg powder, or pressed magnesium powder electrodes are used commonly. Liu R. et al. 2018 by SEM showed no morphology change in Mg foil after and before soaking in electrolyte and because of adsorption of solvent, a layer form on Mg electrode which is good for stability. 10 Sievert B. et al. 2020 showed that pressed Mg powder anode has more surface area than Mg foil, so the possibility of Mg deposition is high, resulting in better charging behavior and higher cycling performance. This anode has a highly porous structure, and magnesium powder's average particle size is 44 μm, so the surface area of this anode is 3000 times more than the Mg foil anode.
Figure 10. The microstructure of the electrode in which the current collector is attached and binder hardens the bonds between the active material (e.g., Sulfur, Magnesium powder) and carbon.
Download figure:
Standard image High-resolution imageNanostructured materials are commonly used because these materials enhance the reversible Mg insertion and extraction reaction and reduce the diffusion of Mg ions. Therefore cathode is made up of carbon and its derivatives or non-carbonaceous compounds like V2O5, Mo6S8, Ti2O, etc. Lighter elements are mainly used as electrode materials due to their higher specific capacities. Variable valence states of transition metal oxide facilitate more electron-storing sites due to which these oxides are generally used as cathode materials, and the electronegativity helps find the types of bonds between transition metal ions and ligands. More electronegativity differences form a more ionic bond. As a result, a dense structure. The electrode's electrochemical potential depends on the valence state, electronegativity, ionic radius, and the local environment of the cations in compounds. Thus small particle-sized transition metal electrodes provide a high surface area which results in high specific energy.
Carbon electrodes are constructed by different arrangements of carbon, such as they form mesopores or micropores structures. Graphene oxide, graphite, or CNTs are also used as electrodes because of the physical properties of materials. As discussed in this section, Fig. 11 gives us a complete microstructural visualization of the sulfur-carbon composite cathode. Zhou J. et al. 2018, presented sulfur@ microporous carbon (S@MC) composite the micropores, provide extensive sulfur loading approximately 64.7 wt % with more active sites for immobilizing sulfur species. In the micropores of carbon, small chainlike S2−4 molecules get highly dispersed inside and ringlike S8 molecules on the outside surface. Microporous carbon has ultra-micropores with median pore width (<0.4 nm) and narrow pore diameters (<2 nm). Better confinement of S2−4 is achieved in this composite; therefore, this cathode exhibits enhanced rate performance and cycling stability in the nucleophilic electrolytes. 10
Figure 11. This is the microstructure of sulfur carbon cathode; sulfur (S8) particles are present in the mesopores of the carbon host material.
Download figure:
Standard image High-resolution imageCarbon nanotubes electrodes also give a conductivity; Kong W. et al. 2015 suggested a polymer encapsulated nano sulfur/super-aligned carbon nanotube (PVP@S-SACNT) composite electrode because of the porous and high conductive structure of the SACNT network and the PVP surface modification, PVP formed a protective layer around SACNTs, and this will suppress the diffusion of polysulphides. As a result, this cathode provides excellent cyclic stability and 100% coulombic efficiency. Thus Mg–S battery performance is the highly dependent microstructure of the electrode.
Now let us see the microstructural changes taking place during a particular battery's operation. First of all, let us talk about the working of an Mg–S battery having a MesoCo@C cathode reported by Sun, J.et al. (2020). The CoSx species plays a significant role in alleviating the shuttle effect by binding with polysulphide species. This CoSx species confined inside the carbon matrix enable surface-catalyzed redox reactions like S/S2 since they have a high binding affinity for polysulphides and high electronic conductivity during the battery's work. The post-discharge XPS analysis of the cathode. The XPS spectra show a splitting of Co 2p spectra into two peaks and a slight shift of Co2+ peak at 781.5 eV and a new peak at 778.6 eV. This current peak of Co(0) was due to the reduction process, and the shifted peak displayed the adsorption of polysulphide. Also, the XPS spectra of S 2p showed a peak at 162.2 eV, which was due to the formation of MgSx species formation during the working of the battery.
23
Further, in Mg–S batteries using organo-sulfur electrodes, the changes happening in microstructure are the breaking of disulfide bonds in the cathode during discharge and the formation of disulfide bonds during the charging process. In DMcT and PAn composite, the redox reactions are fast due to the close contact between these two materials at the molecular level. The PDTDA cathode was better than DMcT and PAn composite because the disulfide bonds were enclosed between polyaniline chains. So it exhibited an intramolecular catalytic effect. The breaking and formation of bonds were easy in this case because the leading polymer chains remained unchanged. The CSM-PAn composite cathode has a coplanar structure with pi-conjugated bonds. The intramolecular reaction of S–S bonds is fast due to planar structure, and there is no change in the leading polymer chains.
17
In Mg–S battery using nitrogen-doped graphene-MWCNT hybrid nanocomposite cathode as reported by Vinayan, B.P. et al. (2019), studied the microstructural changes, especially the formation of polysulphide species during operation. They have used Operando Raman Spectroscopy during the charging and discharging processes. The nitrogen doping in graphene created three functional groups: graphitic, pyridinic, and pyrrolic N. Studies have proven that doping nitrogen creates polarity in the matrix and thus helps bind polysulphides. The bands corresponding to bulk sulfur were observed at 150, 219, and 470 cm−1. During the discharge, these bands decreased and, in the end, wholly disappeared. This behavior might be attributed to the reduction of bulk sulfur to polysulphide chains. The bands for  were maximum between 1.4 to 1.2 V and started decreasing due to further reduction to S2−. This shows the formation of short-chain polysulphides. These results were further validated using XPS spectra. When XPS analysis was done of the new cathode, it displayed two peaks attributed to S8. Further, when the discharged cathode was analyzed, it displayed several low-intensity peaks attributed to other reduced sulfur species. Then all the polysulphide-related peaks in the Raman spectra disappeared, and broad peaks were observed between 180 to 350 cm−1. These peaks confirmed the conversion of the polysulphides to MgS during battery operation. They also concluded that the structure of the final MgS product was of zinc blend phase.
19
were maximum between 1.4 to 1.2 V and started decreasing due to further reduction to S2−. This shows the formation of short-chain polysulphides. These results were further validated using XPS spectra. When XPS analysis was done of the new cathode, it displayed two peaks attributed to S8. Further, when the discharged cathode was analyzed, it displayed several low-intensity peaks attributed to other reduced sulfur species. Then all the polysulphide-related peaks in the Raman spectra disappeared, and broad peaks were observed between 180 to 350 cm−1. These peaks confirmed the conversion of the polysulphides to MgS during battery operation. They also concluded that the structure of the final MgS product was of zinc blend phase.
19
After understanding the microstructural changes happening during the battery's operation, we explain to you how these changes can sometimes hinder the working of a battery. Wang et al. (2019) presented that by the elemental sulfur reduction, the high-order magnesium polysulfides (MgSx, 3 < x < 8) formed are certainly soluble in ether-based solvents that makes them electrochemically transportable. However, they can relocate concurrently from the cathode to the anode, which results in capacity fading and active material loss. 55 Yang et al. (2019) manifested that the reaction between intermediary polysulfides formed through the discharge and charge process with the Mg anode. Mg−S batteries suffer from the shuttle effect mainly. The decomposition of polysulfides results in never-ending active material loss restricts the cycling stability and decreases the specific capacity of Mg−S batteries. Here, low Coulombic performance suggests that excessive polysulfide shuttling is not the principal reason for the apparent decrease of the capability. 25 Vinayan et al. (2016) conveyed that the increase of charge capacity over the discharge capacity after some initial cycles acts as a parasitic side reaction primarily because of the Mg polysulfide shuttle effect. The voltage figures represent that both the capacity and discharge voltage decrease slowly with an increasing rate, which can be responsible for high kinetic, ohmic overvoltages at high current rates and inactive kinetics of Magnesium with Sulfur. Muthuraj et al., 2019 presented a Dual- doped carbon cloth that affects the intermediate higher-order polysulfides, influences their rapid dissolution, and reduces the shuttle effect. 45 Du et al., 2013 proposed that overcharge behavior and low charge-discharge efficiency of a battery is mainly because of polysulfides shuttle behavior. The main reason behind this might be the electrolyte decomposition and corrosion of the current collector, primarily because of charging cells to above 2.5 V. Mg/S battery set forth varied voltage figures while applying various Mg-ion electrolytes, which shows the crucial roles of electrolytes in the working of Mg–S battery. 48 Du et al., 2017 revealed that fast capacity fading and poor cell reversibility result from the dissolution of greater-order polysulfides that are moderately soluble in carbonate-based solvents. High- order polysulfides are easily dissolved in Glyme-based and dioxolane solvents, still accompanying the greater-order polysulfides drifting between the anode and cathode, leading to the "shuttle effect," which results in the and low Coulombic efficiency and active materials loss. 48 Herein, Fig. 12 explains the development of cathode materials and their microstructure's involvement in improving battery efficiency.
Figure 12. Various development in cathode materials and their microstructure's involvement in improving the battery efficiency.
Download figure:
Standard image High-resolution imageDiscussions
Table V shows the development of various electrodes, non-nucleophilic and nucleophilic electrolytes for magnesium sulfur cells which enhance the electrochemical safety and performance of the battery. At the same time, performing experiments for finding the discharge and charge capacity,coulombic efficiency, potential, and cyclic stability of the magnesium sulfur batteries, different types of diffraction, microscopy, and scanning techniques like SEM, TEM, XPS, XRD, FTIR, EFTEM. Thermo Gravimetric Analysis is carried out by N2 adsorption-desorption experiment to find the sulfur loading on the electrode.
Table V. Various electrodes and electrolytes used in Mg–S battery and their respective properties.
| Optimizat ion strategy | Cathode∣∣An ode | Electrolyte | Highest capacity [mAhg−1] | No of Cycles | Cycling stability/cycled capacity [mAhg−1] | References | |
|---|---|---|---|---|---|---|---|
| Heterostru | Co3S4@MXe | 0.4 M | 1220 | 100 | Only 528 | 61 | |
| ctural constructi on | ne-S∣∣Mg | (MgPhCl)- in THF. | AlCl3 | mAhg−1 retained after 100 | |||
| cycles | |||||||
| Electrode- | V2O5∣∣Mg | 0.5 M | 140 | 40 | 95 | 21 | |
| Electrolyte | Mg(TFSI)2/PC+ | mAhg−1 | |||||
| Interface | H2O | retained | |||||
| after 40 | |||||||
| cycles | |||||||
| Nanostruct ure Constructi on | GrapheneMo S2∣∣Mg nanoparticles | Mg(AlCl2BuEt)2/TH F | 170 | 50 | After 50 cycles 95% capacity retention | 62 | |
| Effective | S@pPAN∣∣M | MBA-(MgCl2)2− | 677.9 | 85 | 75.9% | 63 | |
| CEI layer | g | (AlCl3)2 + LiCl/ | cycles | cycling | |||
| Formation | THF | at 0.1 C | stability | ||||
| after 85 | |||||||
| cycles | |||||||
| Mo6S8 ∣∣ Mg | 0.4 M (PhMgCl)2 − | 123 | 500 | 89 mAhg- | 62 | ||
| AlCl3 in THF | 1 retained | ||||||
| after 500 | |||||||
| cycles | |||||||
| Variation in the crystal form | TiS2 ∣∣ Mg | 0.4 M (PhMgCl2)− AlCl3 in THF | 270 | 40 | Only 24 mAhg−1 retained after 40 cycles | 41 | |
| CNF coated separator | ACCS∣∣Mg foil | 0.4 M MgBhfip in DME Solvent | 1000 (initial discharg e) | 20 | 660 after 20 cycles | 10 | |
| S/C∣∣Mg | BCM | 1081 (Initial discharg e) | 30 | 86.4% capacity, retention after 30 cycle | 57 | ||
| Oxygen vacancies are introduced in cathode | SNVOX -CNT∣∣Mg | APC electrolyete | 120 | 180 | 120 mAh·g−1 after 180 cycles | 26 | |
| CNF as cathode and CNF coating on separator | Preactivated S-CNF∣∣Mg metal | [(HMDS)2Mg]+AlC l3 + MgCl2 in tetraglyme solvent | ∼1200 at C/50 | 20 | 1200 last for 20 cycles | 56 | |
| MgF2 solid electrolyte interface (SEI) layer formation on electrodes | SPAN∣∣press ed Mg powder | 0.8 M Mg[B(hfip)4]2 in diglyme /tetraglyme solvent | 500 at C/30 | 1100 | 300,in the 30th cycle. | 11 | |
| Cu2 S@C∣∣Mg metal | 0.4 M(PhMgCl) 2 - AlCl 3 in 1.0 MLiCl/THF solvent | 399.2 (dischar ge)at 0.05 C | 50 | 150(disch arge) at 0.05 C after 50 cycles | 64 | ||
| Introducti on of graphene based electrode | S-rGO∣∣Mg- C | [(HMDS)2Mg] with AlCl3 | 1024 (dischar ge) | 50 | 236, at the end of 50th cycle | 1 | |
| Use of Cu current collector | S-C composite cathode∣∣ Mg metal | Boron centered anion-based magnesium(BCM) | 722 mAh/g (initial) during discharg e | ∼ 15 cycles at C/50 | Only 620 mAh/g retained after 15 cycles | 65 | |
| Use of non- nucleophil ic electrolyte | S/CMK400 PEG with CMC binder∣∣Mg metal anode | Mg-HMDS in DEGIL | ∼510 mA h/g (initial) during discharg e | Retains reversib ility for over 20 cycles | Only about 200 mAh/ g retained at 20th cycle | 12 | |
| Use of non- nucleophil ic electrolyte | S/CMK400 PEG with PVDF binder∣∣Mg metal anode | Mg-HMDS in TEGIL | 800mAh/g (initial) during discharg e | Retains reversib ility for over 20 cycles | Only about 260mAh/g retained at 20th cycle | 12 | |
| Alleviatin g shuttle effect | MesoCo@C cathode∣∣ Mg foil anode | MMAC−DME electrolyte | 830 mAhg−1 at 0.1 C (initial) during discharg e | Atleast 400 cycles | 280 mAhg−1 at 0.2 C at 400th cycle | 23 | |
| Use of high sulfur conversion electrolyte s | S-C composite cathode∣∣ Mg anode | Mg(TFSI)2 and MgCl2 in TEGDME:DOL electrolyte | 1320 mAh g−1(i nitial discharg e capacity) | At Least 4 cycles | Capacity of ∼640 mAh g−1 at 4th cycle | 13 | |
| Use of Mg powder anode | 50 S-cathode∣∣ Mg PALP anode | 1.2 M DEGIL | 600 Ah/kgSulf ur during first cycle | At Least 100 cycles | The capacity of ∼ 50 Ah/kgS ulfur at the end of 100 cycles | 18 | |
For finding a perfect electrode and electrolyte material comparison between the theoretical and experimental discharge capacity is performed. Yu X. et al. (2016) used a formula to find the theoretical capacity of battery i.e.
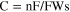
Where C refers to theoretical discharge capacity; F is the Faraday constant; In each electrochemical process, the number of electrons transferred is denoted as n; and FWsrefers to the formula weight of sulfur. 56 Since Mg–S batteries experienced lethargic kinetics during the operation of the battery mainly because of the insulating behavior of sulfur and difficult redox reactions involving polysulphides.
Using a current collector is crucial in Mg–S batteries; copper as a current collector is preferred over other metals. Cu reacts with polysulphide chains to enhance the kinetics. The copper ion generated favors the fragmentation of long-chain polysulphides, and this improves the reaction kinetics. Even the copper sulfide produced at the end can convert into magnesium sulfide due to structural similarity, which means easy extraction of magnesium ions. The cell gives a high capacity of 722 mAhg−1 and is stable even after 12–15 cycles. 65 Other than a current collector, the separator or membrane also plays a crucial role, so in some cases, CNF coating is done on the separator. As a result, this cell shows higher specific capacity than the cell with an uncoated separator. Glass fiber separator or different types of polymer-based separators are used in Mg–S battery. Fichtner M. et al. 2021 reported a glass fiber separator coated with polyoxometalate (POM) clusters/carbon. The polysulfide diffusion was suppressed more effectively than the new glass fiber separators, and the combination of conductive carbon and the POM inhibited the polysulfide shuttle and polysulfide species.reactivation. 66
Among one of the first uses of nucleophilic electrolytes, Zhao-Karger, Z. et al. (2014) revised the old [(HMDS) 2 Mg] based electrolyte reported in previous works and introduced a new kind of non-nucleophilic electrolyte. So this Mg–S cell used a solution of [(HMDS)2 Mg] and AlCl3 in DEG/TEG/DEGIL/TEGIL. Cells using DEG and DEGIL with PVDF binder gave medium capacities, but TEG and TEGIL gave better results, as high as 550 and 800 mAh g−1, respectively. Both CMC and PVDF exhibited medium cyclicity of 260 mAh g−1 for over 20 cycles. To stabilize the cathode and to prevent the corrosion of the anode. The working of an Mg–S battery involves the formation of two voltage plateaus. The high voltage plateau(discharge) relates to the conversion of sulfur to polysulphide, and the low voltage plateau(discharge) relates to the conversion of polysulphides to MgS. The development of modified non-nucleophilic electrolytes like (HMDS)2Mg in ether solutions gave us the liberty to synthesize these electrolytes in various other solvents. Also, it provided good reversibility, oxidation stability, and enhanced ionic conductivity. Ionic liquids as co-solvents with glymes were experimented with and were successful in giving high reversible Mg deposition and suppressed the solubility of polysulphides as they increased the viscosity of electrolytes. However, these have shortcomings, like anodic corrosion due to AlCl3 species, high dissolution of elemental sulfur, and polysulfide dissolution. 12 Despite these technical advances, Lin Sheng et al., 2021 showed that electrolytes consisting of small chain ethers having low boiling points ignite easily and can explode at low temperatures. The mobility of Mg2+ ions is higher in these electrolytes, which boosts the charge and discharge performance. Also, the development of these electrolytes must ensure that they have a higher molecular weight or polarity, therefore obtaining low vapor pressure and high BP. 67 The cycling was much better with ether-based electrolytes than dual-salt polyvalent-metal storage batteries as the electrolyte can decompose quickly. AlCl3 was added to stabilize the anions. Subsequently, Mg2+ intercalates into the FePO4 structure, which destabilizes the structure of LiFePO4. M. Rashad et al., 2020 collectively studied the nucleophilic and non-nucleophilic electrolytes for Mg–S batteries. The nucleophilic electrolytes such as all-phenyl-complex (organohaloaluminate salts (AlCl3, PhMgCl) in tetrahydrofuran) are not favorable as they are corrosive produces large quantities of Mg-PS polysulfides. The performance of these electrolytes was attempted to be improved by a coating of the cathode to prevent the dispersion of polysulfides inside the cathode and the electrolyte by using electrolyte additives such as LiTFSI, LiCF3SO3, tris(2H-hexafluoroisopropanol) borates, iodine (I2), and yttrium chloride (YCl3) salt improve the electrochemical properties of the battery, reduces overpotential and shuttle effect. On the other hand, non-nucleophilic electrolytes B(HFP)3 or [Mg(DG)2] [HMDSAlCl3]2 are compatible, have good cycle stability, lower overpotential values with sulfur-based cathodes. Non-nucleophilic electrolytes are suggested to be used to sense the chemical conversion between sulfur and magnesium sulfide. 68 Glymes with high boiling points and low flammability are suggested to be used; if not, they can cause safety issues. Robba, A. et al. (2017) gave experimental evidence to the formation of two voltage plateaus during the operation of the battery. Their cell used a mixture of MgCl2 and Mg(TFSI)2 in ether solvents as electrolytes. The first discharge capacity was 1320 mAh g−1 and decreased to 630 mAh g−1 by the 4th cycle. The charge process, however, proceeds through a single plateau. Using XANES and RIXS, they determined the process of reduction of sulfur. They prove the consumption of sulfur and the formation of polysulphides during high voltage plateau. In the voltage plateau, the amount of polysulphides decreases while that of magnesium sulfide increases. 13 Due to the high ionic conductivity of Blend polymer electrolytes, it can be used in Mg–S batteries. PVA and PAN composite with 92.5PVA:7.5PAN composition can be used. With the addition of salts in this electrolyte, the conductivity can further increase because the amorphous phase increases and the transport of ions takes place in this phase. 0.3 m.m.% Mg(NO3)2 concentration in BPE film has the maximum amorphous nature. The battery displayed an OCV of 2.02 V and a constant potential of 1.8 V for one month. Which makes this electrolyte suitable for application in Mg- S battery. 14
Many optimization strategies have been developed to explore cathode materials that enhance the electrochemical conductivity and Mg2+ diffusion to augment capacity during the discharge of a battery and rate performance. Cunyuan Pei et al. reported the doping of Mo6S8 by Cu to enhance the electrochemical behavior. Further, it was also noted that the presence of Cu would lead to Cu-Mg repulsion, and it will help improve the reversible capacity. The study also revealed that Mg ions replacement in Ti-vacancies during insertion is very promising. The development of a cathode material with high capacity and high voltage is tricky and difficult. However, the works detailed above have the potential to contribute and help in future directions. The water molecules can protect the Mg2+ ions in the intercalate and can improvise the coulombic interactions, and this interesting result can be very beneficial while using intercalation cathodes to polish up their capacity and rate capability. By augmenting the mobility of Mg2+ ions, alterations in ion transport channels are yet another approach to upgrade the capacity and rate capability. Another approach to developing suitable cathode materials is by using materials that can accommodate Mg2+ ions with charge compensation through electron delocalization rather than metal center reduction. Apart from intercalation cathodes, some efforts are being made in conversion cathodes. This is because of the complexity and problematic Mg- conversion as compared to Li. 62 E. Sheha et al., 2019 introduced orthorhombic V2O5 structure as it is a reversible intercalation cathode for Mg batteries that exhibits high initial capacity. While doping it with S, it exhibited good properties for the accommodation of magnesium ions and its electrochemical performance. 21 The hexamethyldisilazane magnesium chloride was compatible with an Mg/sulfur (S) cell. The electrolyte needs to be compatible with the electrode and to scrutinize that a solution of 0.3 M Mg(TFSI)2 in glyme/diglyme was used as an electrolyte. During the beginning of Mg stripping in Mg/CMK3-S batteries, the discharge rate was kept low at C/200 for five hours to limit the overpotential. Later the rate was increased up to C/100 for initial discharge and C/10 during primary discharge. This battery gave an output of 500 mAh g−1 during the discharge process and a voltage of 0.2 V. They validated the formation of reversible MgS by XRD analysis, as already shown by the above results. Non- corrosive electrolytes based on Mg(TFSI)2 dissolved in diglyme or acetonitrile were used along with Mg2Sn and Mg3Bi2 in cells to examine the Chevrel phase vanadium oxide and Prussian blue analogs. Sulfur-based positive electrodes are one of the best candidates to attain high energy density in Mg batteries. Sun, J. et al. (2020) used an in situ sulfurized carbon confined cobalt cathode(MesoCo@C). This electrode binded the polysulphides to prevent them from shuttling. The cell gave an excellent initial capacity of 830 mAh g−1 at 0.1 C. It displayed a capacity decrease for 100 cycles but then increased, and it was stable at 280 mAh g−1 for at least 400 cycles. 23 Other cathodes such as S@MC composite with 64.7 wt % sulfur content and S@pPAN composite containing 47.3 wt% sulfur restrained the polysulfide dissolution. The highly dispersed small S2–4 inside the carbon micropores and good electrical contact of the composite material with the current collector, i.e., chemical bonding of large S8 molecules with the copper (Cu) current collector, enabled consistent distribution of sulfur in the electrode.By utilizing activated carbon cloth, many sulfur-based cathodes were prepared. These cathodes prepared on mesoporous carbon had a prolonged life cycle and capacity. Many reports have also used carbon nanotubes, porous carbon, reduced graphene oxide, graphene-carbon nanotubes, coating of carbon layer over the cathodes and carbon nanofibres has shown impeccable electrochemical performance in Mg–S batteries. The hierarchical nanostructure or coated carbon layer of MWCNTs has provided a larger electrode/electrolyte contact surface and improved the conductivity. Another cathode material that can minimize the shuttle effect by binding is a nitrogen-doped hybrid nanocomposite of Multi-walled CNTs and graphene. The MWCNTs stacked between sheets of graphene increase the surface area for polysulphide adsorption. Although the discharge capacity of this cell is good, the first cycle capacity is 431 mAh g−1, and 220 mAh g−1 is retained at the 50th cycle; small amounts of sulfur species are present on the cycled Mg anode detected by XPS spectra. 19 In any rechargeable battery, the anode becomes more important because of the possible creation of an anode-shielding layer which can hinder the reversibility of the battery.These sulfur species form a blocking layer on the anode due to the formation of MgS. This passivation layer is not good for the electrochemical results of the battery. Also, not much attention has been given to the Mg anode to find a proper anode-cathode combination. Therefore, Sievert, B. et al. (2017) has used an Mg–S battery with powdered anode and distinguished the performance from Mg- foil anode. A PALP anode battery gave a capacity of 600 mAh g−1 after the 1st cycle itself.However, the discharge capacity decreases to nearly 100 mAh h−1 nearing the end of the 100th cycle. While a PAHP anode battery has a very less discharge capacity during the first cycle, approximately 350 mAh g−1, and reduces up to approximately 80 mAh g−1 after the 100thcycle. Also, the foil anode gave a discharge capacity of 350mAhg−1 during the 1st cycle and approximately 130 mAhg−1 at the end of the 13th cycle. Thus, a powdered anode PALP gave better results than a foil anode. 18
Process parameters: a guide to accuracy
The below-discussed process parameters are instructions to guide the researchers in the field to obtain an efficient result using the outcomes we have discussed in the literature survey. The key findings of the literature are provided in "Discussions," which explains why these parameters are optimum.
- 1.The presence of a binder on an electrode highly influences the battery's performance because the physical properties of electrodes are remarkably affected. Various polymeric binders that are organic or water-soluble are commonly used, but the sodium polyacrylate(PAAS)binder with sulfurized pyrolyzed polyacrylonitrile (S@pPAN) composite cathode show significantly high capacity retention and cyclic stability due to its outstanding cohesion and adhesion property.
- 2.The electrolyte's compatibility with the sulfur and Mg electrodes is a foremost concern while constructing a reversible magnesium battery. Most of the electrolytes contain AlCl3, which is corrosive to the electrodes. Boron centered magnesium electrolyte in DME solvent show excellent performance as it is non-corrosive and shows non- nucleophilic characteristics hence highly compatible with the electrophilic sulfur. Research shows that for more than 100 cycles, organic magnesium borate-based electrolyte offers a stable discharge capacity above 1000 mAhg−1, and this electrolyte is synthesized efficiently compared to another non-nucleophilic electrolyte.
- 3.The doping of ions can improve the Mg–S battery electrochemical performance, nanostructure construction, fabrication of the prepared composites, change in the crystal structure, electrode-electrolyte contact enhancement makes Mg-deposition and stripping more straightforward on Mg anode and also are proved to be efficient modification methods.
- 4.The non-nucleophilic electrolytes are found to be more compatible, non-corrosive when electrolyte additives are added to boost the functioning of the battery. Also, improved cycle life with sulfur-based cathodes than the nucleophilic electrolytes.
- 5.Using a current collector can give a revamped performance for an Mg–S battery, but it depends on the selection of metal as a current collector. In the same electrolyte, cathode, and anode, Aluminium as current collector shows inferior electrochemical performance than copper, with large overpotentials and low capacities. Therefore, copper as a current collector can result better by promoting the segmentation of polysulphide chains due to copper-polysulphide reactions.
- 6.A combination of Mg(TFSI)2 and MgCl2 salts in 1:1 ratio in dioxolane and tetraglyme as an electrolyte gives the highest first discharge capacity(1320 mAh g−1) compared to other non-nucleophilic electrolytes previously reported like Mg-HMDS based electrolytes in THF and glymes and BCM electrolyte. Potential during first discharge is low due to the passivating film present on the anode surface, but it increases in further cycles after activation of the anode surface.
- 7.The mobility of Mg2 + ions is enhanced, and charging-discharging results are also improved by using electrolytes consisting of small chain ethers with low boiling points and blowing-up temperatures.
- 8.
Conclusions
To summarise, it can be said that Mg–S batteries have seen some incredible developments concerning electrolytes, electrodes, or separators. There is growth in both non-nucleophilic and nucleophilic electrolytes. In the Mg–S battery, to prevent any side reactions between the sulfur electrode and electrolyte, a non-nucleophilic electrolyte is required. Various non- nucleophilic electrolytes have been developed till now that are compatible with sulfur cathodes and Mg anode. Moreover, we have widely discussed electrolyte additives, sulfur composite-based cathodes, improved cell configurations, cathode materials for the Mg–S system, different anode materials apart from Mg metal. Among the various cathodes, graphene and mesoporous carbon electrode showed the best electrochemical performance. However, the Mg–S battery still has a drawback of shuttling soluble polysulphides during the battery's operation. This leads to the total blockage of metal anode. The battery suffers capacity fading due to active material loss and very low cyclicity in some cases. The performance of any Mg–S battery depends on the combination of electrolyte, anode, and cathode. Therefore, the electrochemical performance varies from battery to battery. We have already seen various electrolytes and electrodes that enhance or affect Mg–S batteries' capacity during dynamic conditions. However, the Mg–S battery also suffers self-discharge during static conditions arising because of polysulphide migration, resulting in anodic resistance due to the formation of the passivation layer over time. Studies suggest that more or less every electrolyte suffers self-discharge.To sum it all up, we have put forward the following concluding remarks.
Conclusions
In this review, we have discussed reaction mechanisms, working, and construction of Mg–S batteries. We have widely discussed different cathode materials used in the battery that can achieve high energy density and specific capacity. Some critical challenges which still exist are the relatively low areal capacity, the low discharge potential, slow ion transfer, the continuous deposition of Mg2 + ions causes the formation passivation layer on the Mg anode surface, which block the electrochemical reactions and the diffusion pathway of Mg2 + at room temperature, the high electrostatic interaction and divalent nature of magnesium can lead to slow and lethargic kinetics of magnesium-based battery and shuttling effect of polysulfides, etc. Many conventional electrolytes and electrodes are incompatible when used in Mg–S batteries. However, these challenges can inspire future research, leading to more advanced discoveries of battery configurations, battery materials, and accompanying battery chemistries. This review includes the solutions to many such challenges faced while working with magnesium-sulfur batteries and which tend to provide a way to improve the battery's overall performance. Some of them include the structural and constructional changes in the design of sulfur hosts as cathodes to attain better performance, use of high capacity anode materials other than the commonly used Mg metal as anode provides a conductive and protective layer on the metal anode surface.
Moreover, to prevent the self-discharge of Mg–S battery and to enhance their shelf life, electrolytes preventing or reducing the solubility should be developed, or the contact of these species with metal anode should be mitigated using an ion-selective coating, artificially prepared magnesium SEI( solid electrolyte interphase) or any other method. The increasing sulfur loading in the sulfur-based composite cathodes and decrease in the E/S ratio are suitable approaches to achieve high energy densities of Mg–S battery. Also, to avoid the complete and total blockage of magnesium anode due to the reactions of polysulphides, an in situ-electrochemical activation of metal anodes is a potential solution. In this work, the development of carbon host material was widely adopted for Mg–S batteries because of its high electrical conductivity, robust chemical pinning-down of sulfur and successively formed polysulfides, increased accessibility of the liquid electrolyte to the sulfur, a super stable skeleton to accommodate the strain produced due to the alterations in the volume of sulfur during the cycling, and tiny pores without enlarged openings to take in polysulfides. Out of various carbon nanomaterials, the most desirable materials for energy storage are grapheme based nanostructures because of their distinctive properties like high charge mobility, large surface area, outstanding thermal and electronic conductivity, and good chemical stability and high mechanical strength. Therefore, along with developing cathodes that encapsulate the polysulphide species, further research should focus on developing electrolytes that can enable this process or some additives that can change the interface properties. In the battery, after the first cycle, the Mg anode gets conditioned, and during the further cycles, it gives a high discharge voltage. The interface properties of the anode get changed, as this layer made up of magnesium salts and other species prevents the passivating species from getting in contact with the anode surface. The use of LISICON can prevent the reaction of magnesium with oxygen, carbon dioxide, and water, which formed a layer on magnesium and prevented further reaction. The use of lithium intercalation compound solves the difficulty of Mg2 + intercalation so to so to previous, which is a slow process. Presently, some issues associated with the possibility of practical applications of solid-state electrolyte (LISICON) are to be dealt with appropriately. Reasons behind those problems are-
- (1)Primary materials required for its manufacture are not expensive, but its cost is high, so cheaper methods are needed for its manufacturing.
- (2)At room temperature, the ionic conductivity of LISICON is not that high and huge overpotentials are produced.
Many pieces of research are underway for this, which might result in a good energy storage system. Furthermore, expansion and progress in the hybrid energy system with supercapacitors and other energy storage devices for future electric vehicles and portable electronic appliances will be a major subject in the upcoming years. 4,34–37,91,34–37 92–104
Acknowledgments
Authors would like to thank Prof. Dr. Sanjeevikumar Padmanaban, Aarhus University, Denmark, who has guided the authors. The authors would also present gratitude for Prof. Dr. Murali Banavoth, University of Hyderabad, and Prof. Dr. Prashant Sonar, Queensland University of Technology, Australia to guide and provide important information for this review. The authors would like to thank Prof. Dr Kathiravan Srinivasan for his kind support. The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions
Conceptualization, UC and PB; methodology, UC, PB, SP; software, UC, and PB; validation, SP; formal analysis, UC, PB, SP, DK, GP, SN, MS, MB, PS, SS, SL, AKR, BB, and NSR; investigation, UC, DK, GP, SN, MS, MB, and PS; resources, UC, DK, GP, SN, MS, MB, PS, SS, SL, AKR, BB, and NSR; data curation, UC; writing—original draft preparation, UC, DK, GP, SN, MS, MB, PS, SS, SL, AKR, BB, and NSR; writing—review and editing, UC, PB, MB, PS, SS,; visualization, UC, and PB; supervision, SP; project administration, UC, PB, and SP; funding acquisition, SP. All authors have read and agreed to the published version of the manuscript.