Abstract
A combination of a Li4Ti5O12 (LTO) anode and 4 V-class cathodes has been electrochemically studied with a view to its adoption for 12 V-class bipolar batteries. Five series-connected LTO/LiMn0.85Fe0.1Mg0.05PO4 (LMFP) cells harmonized well with a useable voltage range of 12 V lead-acid batteries, which is suitable for low-voltage system applications. The LMFP cathode had excellent cycle life performance during high-temperature cycling at 60°C and over-discharge cycling tests. In the case of the LTO/Al and the LMFP/Al electrode using an Al current collector in a hybrid solid electrolyte consisting of a cubic garnet-type Li7La3Zr2O12 and a gel polymer, lithium insertion and extraction occurred smoothly without irreversible reactions in the potential range of 1 to 4.5 V vs. Li/Li+. The thin hybrid solid electrolyte with thickness of a few micrometer exhibited not only high-rate discharge but also a low self-discharge for practical use. It was demonstrated that the fabricated 12 V-class bipolar LTO/LMFP battery with a capacity of 102 mAh had the average discharge voltage of 12.5 V, the energy density of 90 Wh kg−1, and the output power density of 1500 W kg−1 for 10 s. The 12 V-class bipolar LTO/LMFP battery is expected to be suitable for low-voltage systems.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Lithium-ion batteries using Li4Ti5O12 (LTO) anode realize high power, long life, and safety for automotive and stationary power applications because the LTO electrode has the advantages of nano-size particle, no lithium plating at quick-charging and in low-temperature conditions, outstanding safety for internal short-circuit, and thermal stability under high-temperature conditions.1–5 There have recently been growing expectations, that the LTO-based lithium-ion batteries will be an excellent match for low-voltage system applications such as 12 V start-stop systems for vehicles5 and the replacement of lead-acid batteries for stationary power supply.6,7 The start-stop batteries will be stressed by repeated starting and out-put high-power for cold-cranking operation. The LTO-based batteries may solve these problems and have good voltage harmonization with lead-acid batteries. 12 V-class batteries consisting of five series-connected LTO-based cells with LiMn2O4 cathode have already been applied to start-stop vehicle systems.5
We have been developing olivine LiMn1-xFexPO4 (LMFP) materials as promising cathodes for large-scale 12 V-class batteries in view of the excellent safety and life performance because the LMFP has high thermal stability compared to that of 4 V-class cathodes such as LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4.8 Lithium-ion cells with a combination of the LMFP cathode and the LTO anode exhibited a voltage plateau of about 2.5 V for Mn3+/Mn2+ redox, excellent safety, and high-energy density compared to LTO/LMO cell, indicating the possibility of promising batteries for 12 V lead-acid replacement batteries and stationary power sources.8,9
We have focused recently on bipolar battery technologies in order to make 12 V battery systems simple and low-cost compared with the battery module. Therefore, we conducted a preliminary study of a hybrid solid electrolyte based on lithium-ion conducting ceramics with a gel polymer electrolyte in order to overcome the issues of all-solid electrolyte batteries and we fabricated 12 V-class bipolar LTO/LMFP batteries.10 The bipolar batteries generally have the advantages of simple battery structure, size reduction, and low-cost. In addition, LMFP cathode and LTO anode share the same aluminum current collector, which makes the bipolar structure even simpler. We have developed the hybrid solid electrolyte composed of a cubic garnet-type Li7La3Zr2O12 (LLZ) and a small amount of polyacrylonitrile (PAN) gel polymer electrolyte formed as a very thin layer between the LTO anode and the LMFP cathode with no liquid junction in the bipolar battery. Development of hybrid solid electrolytes is necessary in view of the desirability of high ionic conductivity, low interface resistance between the electrode and the electrolyte, and high mechanical strength for high-rate, low-temperature discharge performance, and low self-discharge. In this study, we report the electrochemical characteristics of LMFP cathode and LTO/LMFP cells using the hybrid solid electrolyte. The preliminary and basic performance of liquid-free and separator-free fabricated bipolar LTO/LMFP batteries was investigated for 12 V low-voltage system applications.
Experimental
Mg-doped LiMn0.85Fe0.1Mg0.05PO4 was used as the LMFP cathode because the discharge capacity was 158 mAh g−1 compared to 145 mAh g−1 for LiMn0.85Fe0.15PO4.8 LiMn0.85Fe0.1Mg0.05PO4 powder was synthesized by using a hydrothermal process. Lithium, manganese, iron, and magnesium sulfates, diammonium hydrogen phosphate, and carboxymethylcellulose (CMC) were used as starting materials. The materials with purified water in the autoclave were heated at 200°C for 3 hours. The obtained powder was pulverized by a ballmilling process. The milled powder was heated at 700°C under a flow of Ar containing 3 vol % H2 for 1 hour.8,11 The hydrothermal process is a promising method for synthesizing highly crystalline particles. LMFP secondary particles with an average single particle size of about 80 nm and no impurity phase were obtained. The other cathode of commercial LiMn2O4 (LMO), LiCoO2 (LCO), and LiNi0.5Co0.2Mn0.3O2 (NCM) samples were used as received. Spinel Li4Ti5O12 power for the LTO anode was obtained by a conventional solid-state reaction method. A mixture of stoichiometric quantities of raw materials such as Li2CO3 and anatase-type TiO2 was heat-treated at approximately 800°C in an air atmosphere. The product was ground to obtain fine powder with an average single particle size of about 0.9 μm. The LTO anode and the cathode construction were the same as those described previously.4,5 The organic liquid electrolyte consisted of a mixture of propylene carbonate (PC) and diethylcarbonate (DEC) solvent (1:2 by volume) containing 1.2 mol dm−3 lithium hexafluorophosphate (LiPF6). The LLZ-based hybrid solid electrolyte with PAN-based gel polymer electrolyte prepared by the method described in a previous report10 consisted of 96 wt% LLZ, 0.8 wt% PAN, 0.6 wt% LiPF6, 0.8 wt% PC, and 1.7 wt% DEC. The PAN-based gel polymer electrolyte was prepared from a mixture of 2 wt% of PAN-based monomer and 98 wt% of electrolyte composed of PC and DEC (1:2 by volume) containing 1.2 mol dm−3 LiPF6 in a polyethylene (PE) separator (20 μm thickness), and then heated at 60°C for 20 h to complete the crosslinking polymerization.
Li7La3Zr2O12 (LLZ) powders were synthesized by a solid-phase process at 980°C using dried Li2CO3 (99% purity), La2O3 (99.99% purity, pre-dried at 900 °C for 12 h) and ZrO2 (99% purity) as reagents. 15% excess of Li2CO3 was added to the stoichiometric ratio to compensate for Li loss during the synthesis. Synthesized particles were crushed to an average single particle size of 200 to 400 nm by beads milling. These materials were subjected to X-ray powder diffraction to confirm crystal phase formation.
Charge-discharge tests and cyclic voltammetry of active materials of LTO, LMFP, NCM, and LCO were carried out using a three-electrode glass cell with a lithium metal foil counter electrode, a lithium metal chip reference electrode, the PE separator, and the electrolytes. Small-sized LTO-based cells using various cathodes were used to investigate charge-discharge characteristics, discharge rate, self-discharge, and cycle performance. The discharge rate capabilities of the small-sized LTO/LMFP cells with the LLZ-based hybrid solid and the PAN-based gel polymer electrolyte were measured at 25°C by various currents from 0.2 C to 8 C rate down to 1.5 V after CC-CV charging at 0.2 C up to 2.7 V to 0.05 C. Self-discharge tests of LTO/LMFP cells stored at 45°C after charging up to 2.7 V were carried out by measuring the residual capacity after storage for 7, 14, 28, and 90 days compared to that of a conventional lead-acid battery.
Laminated LTO/LMFP and LTO/LMO cells with a capacity of 1 Ah constructed by using the LTO anode, LMFP and LMO cathodes, PE separator, and the liquid electrolyte were used to investigate high-temperature cycle performance and the dissolution amount of Mn from the cathodes in the cells stored at high temperatures. Charge-discharge curves at 0.2 C rate with open-circuit voltage (OCV) of 5 series laminated cells were measured between 13.5 V and 7.5 V compared to those of a 12 V lead-acid battery. The charge-discharge cycling tests of the laminated cells were carried out between 2.7 and 1.5 V at 60°C. The dissolution amount of Mn from LMFP and LMO cathode to the electrolyte in LTO/LMFP and LTO/LMO cell stored at 55°C for 1 week after charging up to 2.7 V was measured by Flame-Less Atomic Absorption Spectrometry (FL-AAS).
12 V-class bipolar LTO/LMFP batteries were constructed by the method described in a previous report.10 Figure 1 shows the cross-sectional image of bipolar LTO/LMFP battery. The LLZ-based hybrid solid electrolyte layer was very thin, with a thickness of a few micrometers, compared with a conventional separator. LTO anode and LMFP cathode shared the same Al current collector, which is suitable for constructing simple bipolar batteries. The charge-discharge characteristics, discharge rate, and low-temperature performance of 12 V-class bipolar batteries were measured between 13.5 and 7.5 V. Power densities of output and input for 10 s were calculated from the results of Hybrid Pulse Power Characterization (HPPC) test.12 Charge-discharge cycling tests of 5 V-class and 12 V-class bipolar batteries were carried out between 5.4 and 3.0 V and between 13.5 and 7.5 V at 2 C rate and 25°C, respectively.
Figure 1. SEM image of cross-section of bipolar LTO/LMFP battery with LLZ-based hybrid solid electrolyte layer.
Results and Discussion
Properties of LMFP cathode for 12 V battery systems
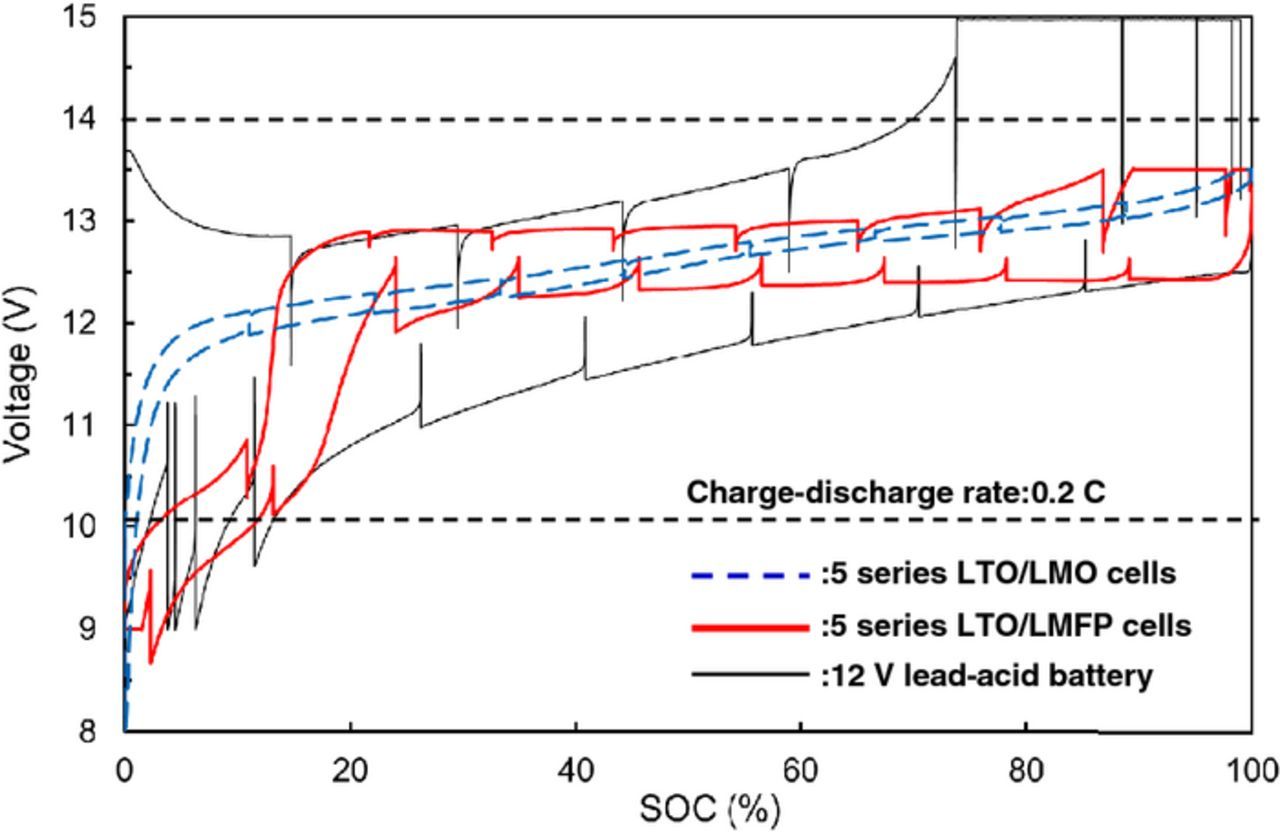
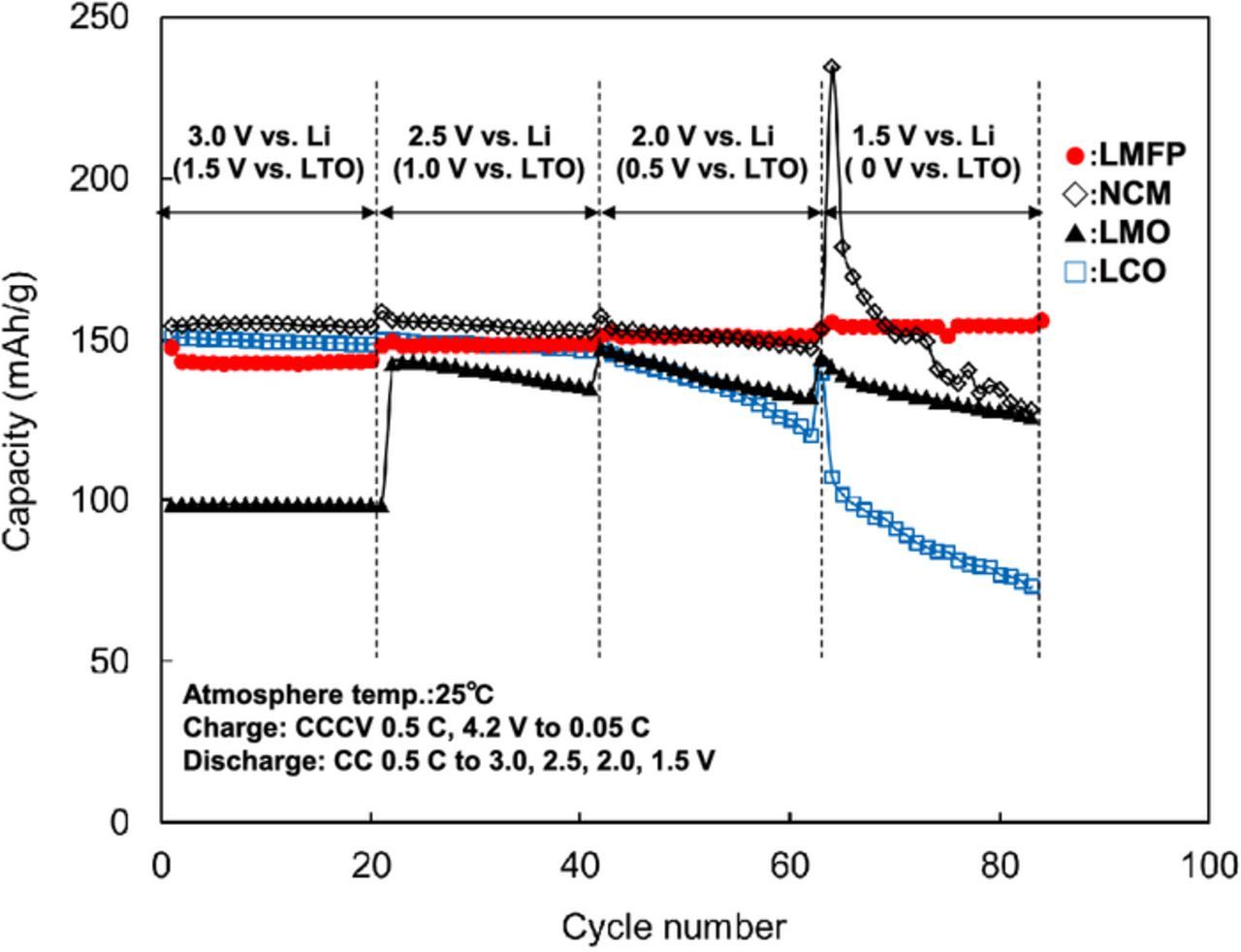
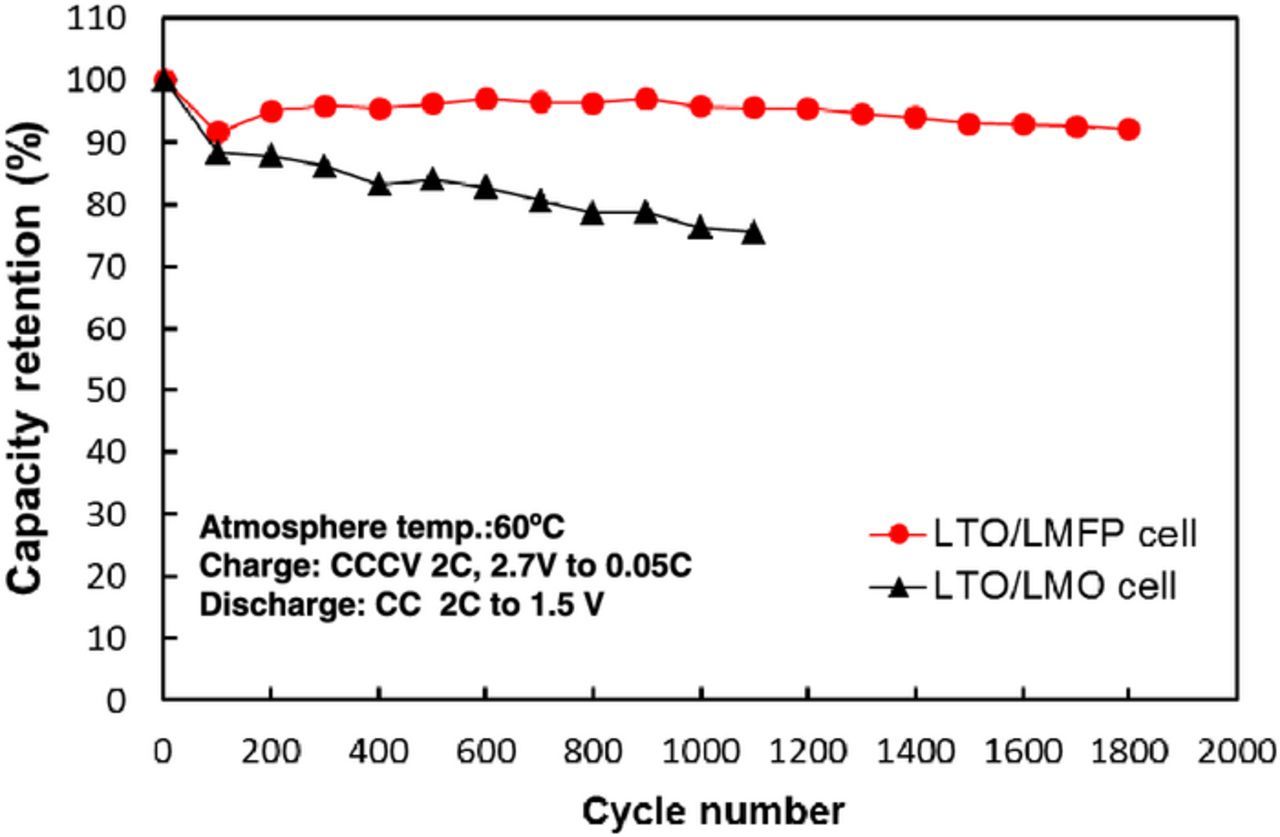
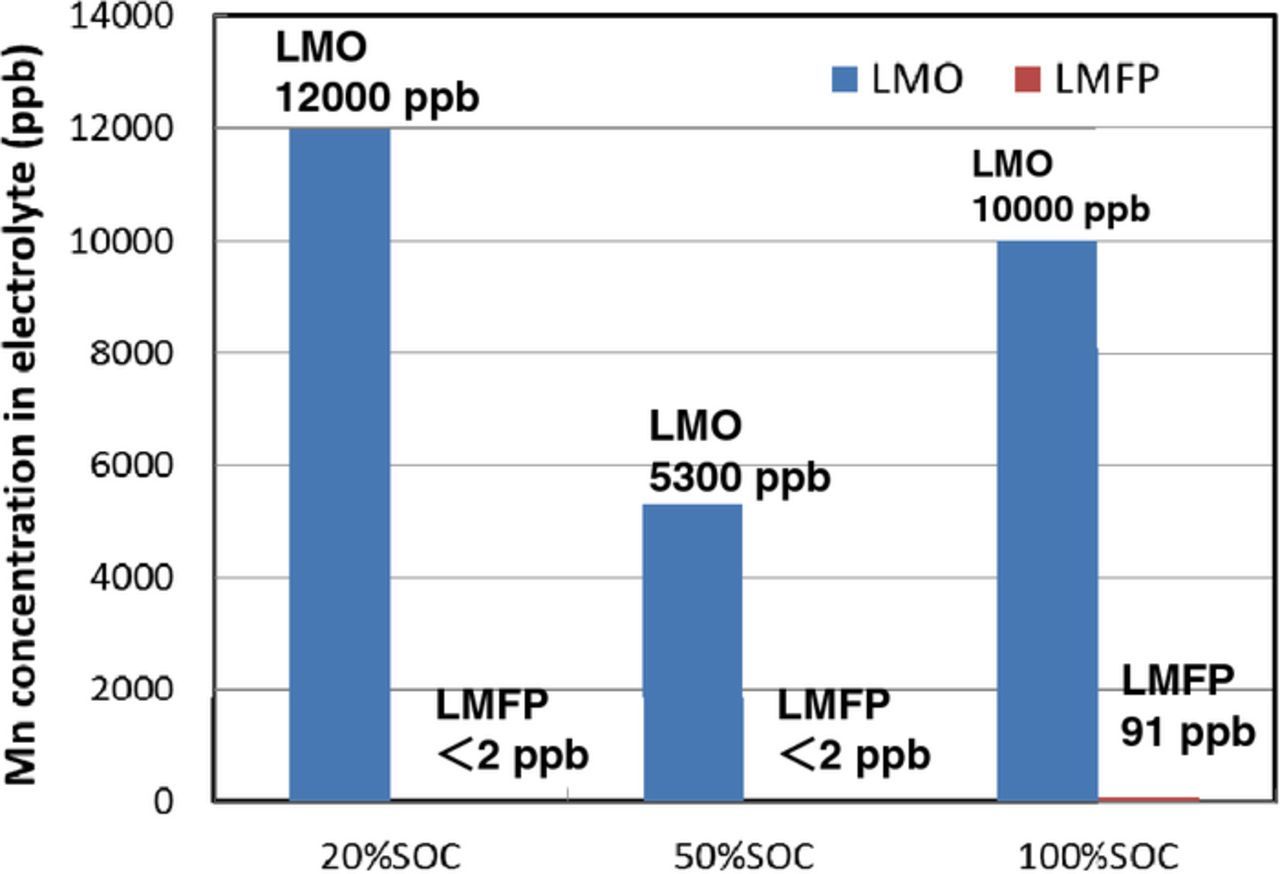
We have investigated electrochemical characteristics and stability of 4 V-class cathodes in combination with LTO anode for 12 V-class low-voltage systems. The average voltages of LTO-based cells using NCM, LMO, and LMFP, cathode were 2.2, 2.5, and 2.5 V, respectively. Hence, five series-connected LTO/LMO and LTO/LMFP cells are considered to be suitable for 12 V-class batteries. The discharge capacity of LMFP cathode was 40% larger than that of LMO cathode. Figure 2 shows charge-discharge voltage curves at 0.2 C rate and open-circuit voltage (OCV) of five series-connected LTO/LMO and LTO/LMFP cells, and 12 V lead-acid battery. The spikes in the charge-discharge curve are OCV data. The LTO/LMO and LTO/LMFP cells were operated between 10 and 14 V, in the useable voltage range of lead-acid battery. The polarizations of LTO/LMO and LTO/LMFP cells were smaller than that of lead-acid battery. The LMO cathode had a very small polarization, which is suitable for high-power applications.5 The LMFP cathode will be suitable for large-capacity battery systems rather than high-power battery systems.8,9 Several cathodes in a number of series-connected cells may deeply discharge by an increase in the capacity difference of cells as the charge-discharge cycling proceeds. Over-discharge cycle performance of 4 V-class cathodes is shown in Figure 3. The LMFP cathode showed no capacity fading during over-discharge cycles between 2.5 and 1.5 V vs. Li/Li+ corresponding to 1.0 and 0 V vs. LTO anode, respectively, which supports that Jahn-Teller distortion is not responsible for the cycle performance of LMFP cathode in contrary to LMO cathode.13 The capacities of LMO, NCM, and LCO cathode significantly decreased during over-discharge cycling. The LMFP cathode was superior to other 4 V-class cathodes in view of the over-discharge conditions, indicating that olivine structure is electrochemically stable in a wide voltage range. We have also investigated durability of LMPF cathode under high-temperature conditions. It is well known that Mn from spinel LiMn2O4 cathode materials dissolves into the electrolytes and is deposited on the graphite anode under high-temperature conditions.14,15 Figure 4 shows high-temperature cycle performance of laminated LTO/LMFP and LTO/LMO cell with a capacity of 1 Ah at 60°C. The LTO/LMPF cell maintained high-capacity retention of 92% at 1800 cycles. It was previously reported that the cycle stability of LMFP cathode at 60°C was significantly improved by the effect of Fe2+ substitution.16 The capacity retention of LTO/LMO was less than 80% at 1000 cycles. The LMFP cathode had excellent high-temperature cycle performance compared to LMO cathode. Mn dissolution amounts from LMFP and LMO cathode to the electrolyte in the laminated LTO-based cells stored at various States of Charge (SOC) and 55°C for one week are shown in Figure 5. Mn dissolution of LMFP cathode in LTO-based cell was two orders of magnitude smaller than that of LMO cathode. We consider that LiMn1-xFexPO4 cathodes in the electrolytes containing LiPF6 salt with an impurity such as HF acid under high-temperature conditions are chemically stable compared to spinel LiMn2O4 with Mn dissolution reactions in the electrolytes.17 The LMO cathode showed the largest dissolution amount of Mn at a low SOC of 20%, which causes the capacity fading to accelerate during the high-temperature cycling as shown in Figure 4. Improvements of high-temperature life of LMO cathodes have been widely studied, which involve protective oxide coating on the surface and additives to the electrolytes, but are insufficiently practicable for use under high-temperature conditions. Therefore, LTO/LMFP cell systems with good durability against over-discharging and high-temperature cycling indicate the possibility of promising batteries for low-voltage system applications such as a replacement of 12 V lead-acid batteries.
Figure 2. Charge-discharge curves at 0.2 C rate and OCV of five series LTO-based cells with LMO and LMFP cathode and 12 V lead-acid battery.
Figure 3. Over-discharge cycle performance of 4 V-class cathodes in three-electrode glass cells.
Figure 4. Change in capacity retention during charge-discharge cycling of LTO/LMO and LTO/LMFP cell at 2 C rate and 60°C.
Figure 5. Mn dissolution data from LMO and LMFP cathodes in the 1 Ah cells with 1.2 M LiPF6-PC/DEC(1:2) electrolyte stored at 55°C for 1 week.
LTO/LMFP cells using LLZ-based hybrid solid electrolyte for bipolar batteries
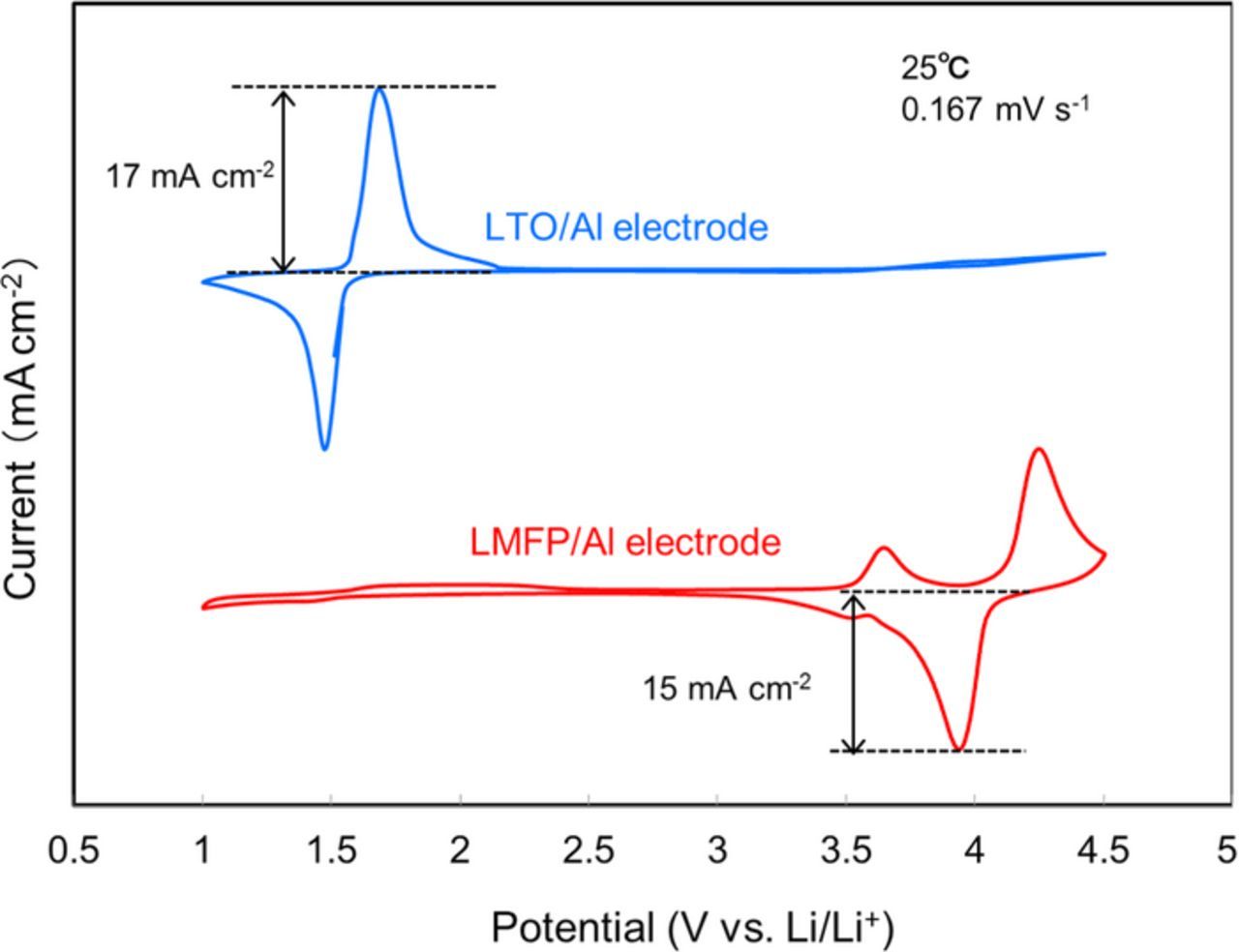
In order to realize 12 V-class bipolar LTO/LMFP batteries, we have developed a thin LLZ-based hybrid solid electrolyte.10 Figure 6 shows cyclic voltammograms of LTO/Al and LMFP/Al electrode with the Al current collector in the LLZ-based hybrid solid electrolyte at a sweep rate of 0.167 mV s−1. In the case of the LTO and the LMFP, lithium insertion and extraction occurred without irreversible reactions such as decomposition of the electrolyte and dissolution of the Al current collector in the potential range of 1.0 to 4.5 V vs. Li/Li+. The hybrid solid electrolyte was electrochemically sufficiently stable to use the LTO anode and the LMFP cathode. If the potential of LTO anode goes up to 4.5 V and that of the LMFP cathode goes down to 1.0 V, the over-discharge of the LTO/LMFP cell will be −3.5 V but may not cause major damage to cell performance. Bipolar LTO/Al/LMFP electrodes have good durability against even such a deep over-discharge, which suppresses the capacity fading during the over-discharge cycles caused by the difference of electrode capacities.
Figure 6. Cyclic voltammograms of LTO/Al and LMFP/Al electrode with LLZ-based hybrid solid electrolyte in the potential range between 1.0 and 4.5 V vs. Li/Li+.
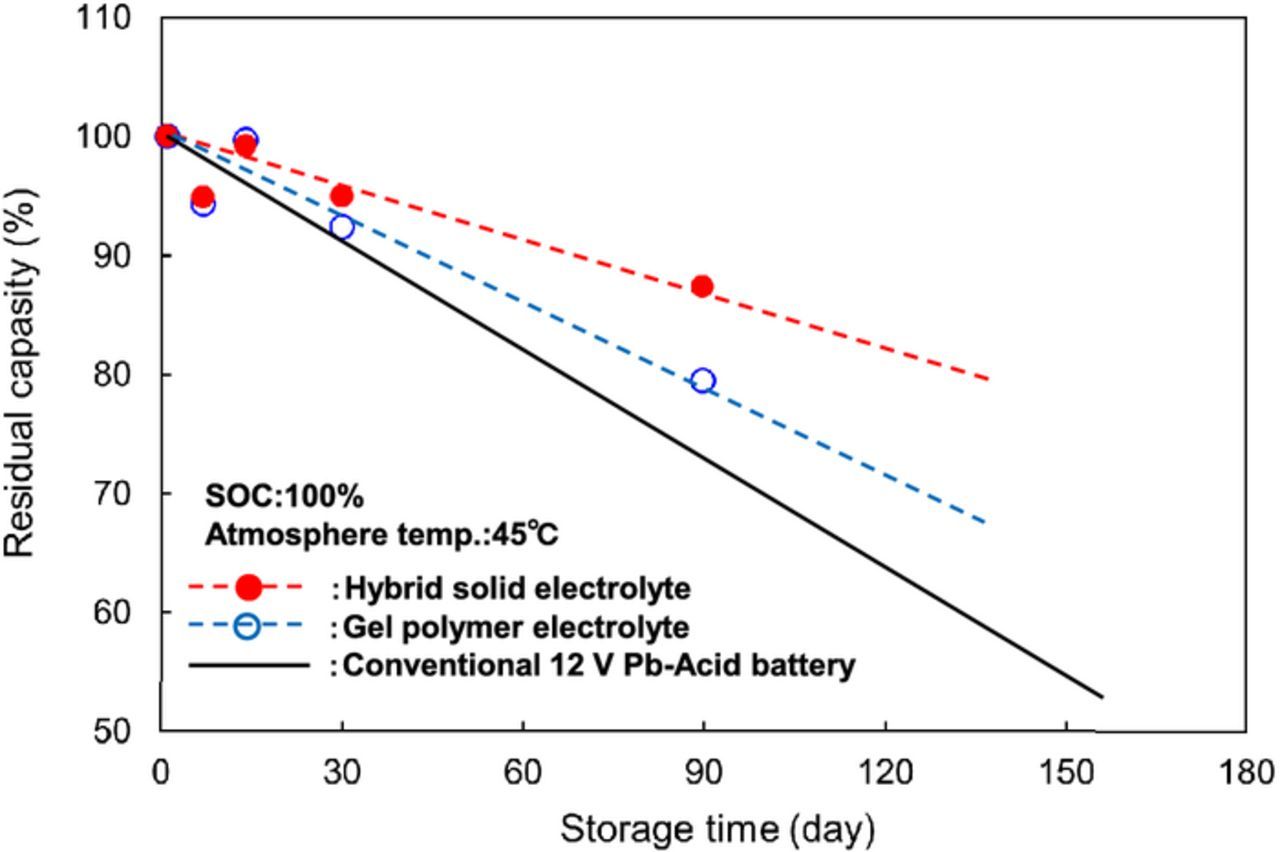
Figure 7 shows discharge rate capabilities of LTO/LMFP cells using the LLZ-based hybrid solid electrolyte and the PAN-based gel polymer electrolyte with the PE separator (thickness:20 μm). The LTO/LMFP cells maintained the discharge capacity retention of about 80% at 8 C rate, which indicates good discharge rate performance in spite of low ionic and electronic conductivities of LMFP compared to those of LiFePO4. Some groups reported that the performance of LiMnPO4 cathode was improved by Mg doping and carbon coating.18,19 We consider that the good performance is mainly attributed to using the hydrothermally synthesized LMFP by Mg doing and carbon coating as the cathode. Discharge rate performance of the cell using the LLZ-based hybrid solid electrolyte even with 4 wt% PAN-based gel polymer electrolyte was slightly superior to that of the cell using the PAN-based gel polymer electrolyte. Such a good discharge performance is attributed to use of the thin hybrid solid electrolyte with a thickness of 3–8 μm. We investigated an influence of the thin hybrid solid electrolyte on self-discharge for long-term storage because thin separator generally accelerates self-discharge. As shown in Figure 8, residual capacity of the LTO/LMFP cell using the thin hybrid solid electrolyte for the long-term storage of 90 days at 45°C was higher than that of the cell using the thinner gel polymer electrolyte and conventional 12 V lead-acid battery, which indicates no acceleration of self-discharge by using thin hybrid solid electrolyte. We consider that LLZ particles have only lithium-ion conducting without side reactions related with anions of PF6− and F−, electrolyte solvents, and impurities such as H+, CO, and CO2 on the cathode and the anode. Therefore, the thin electrolyte layer consisting mainly of LLZ solid electrolyte suppresses the self-discharge reactions. The values of self-discharge rate for LTO/LMFP cell may be larger than those of conventional Li-ion batteries using other cathodes such as LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 because the specific surface area of LMFP powder is much larger than those of other cathodes. We are now developing LMFP cathodes with the small surface areas comparable to those of other cathodes.
Figure 7. Discharge rate performance of LTO/LMFP cells using LLZ-based hybrid solid and PAN-based gel polymer electrolyte at 25°C.
Figure 8. Self-discharge performance of LTO/LMFP cells using LLZ-based hybrid solid and PAN-based gel polymer electrolyte stored at 45°C after charging up to 2.7 V.
Performance of fabricated 12 V-class bipolar LTO/LMFP battery
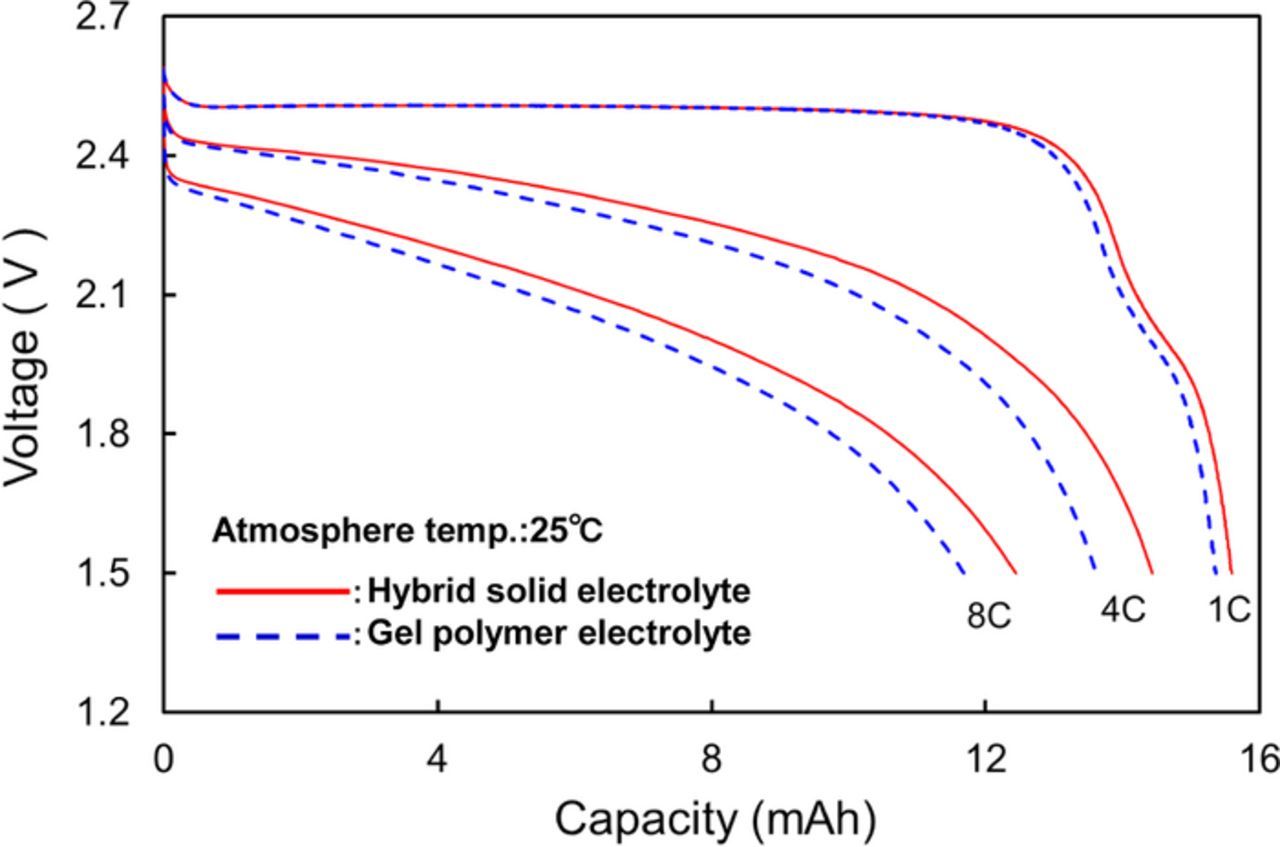
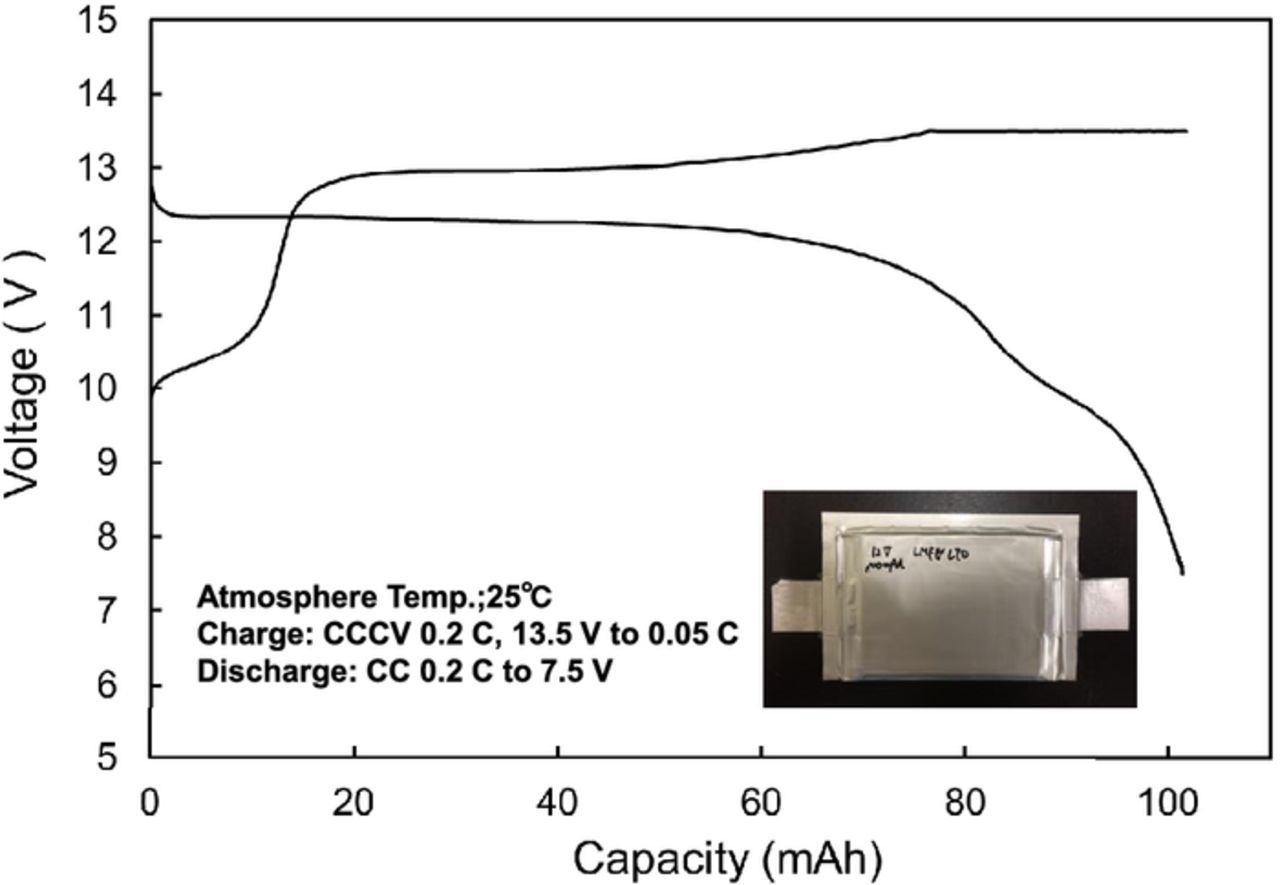
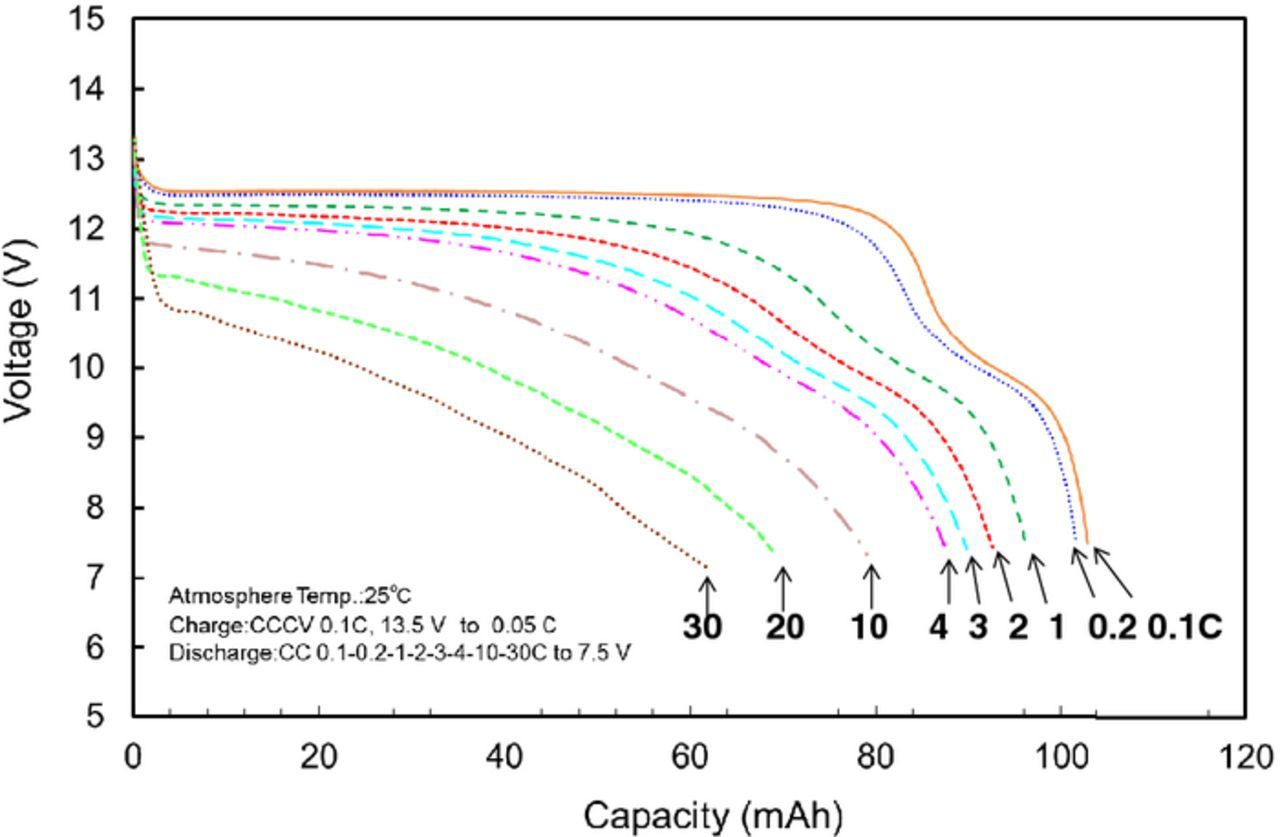
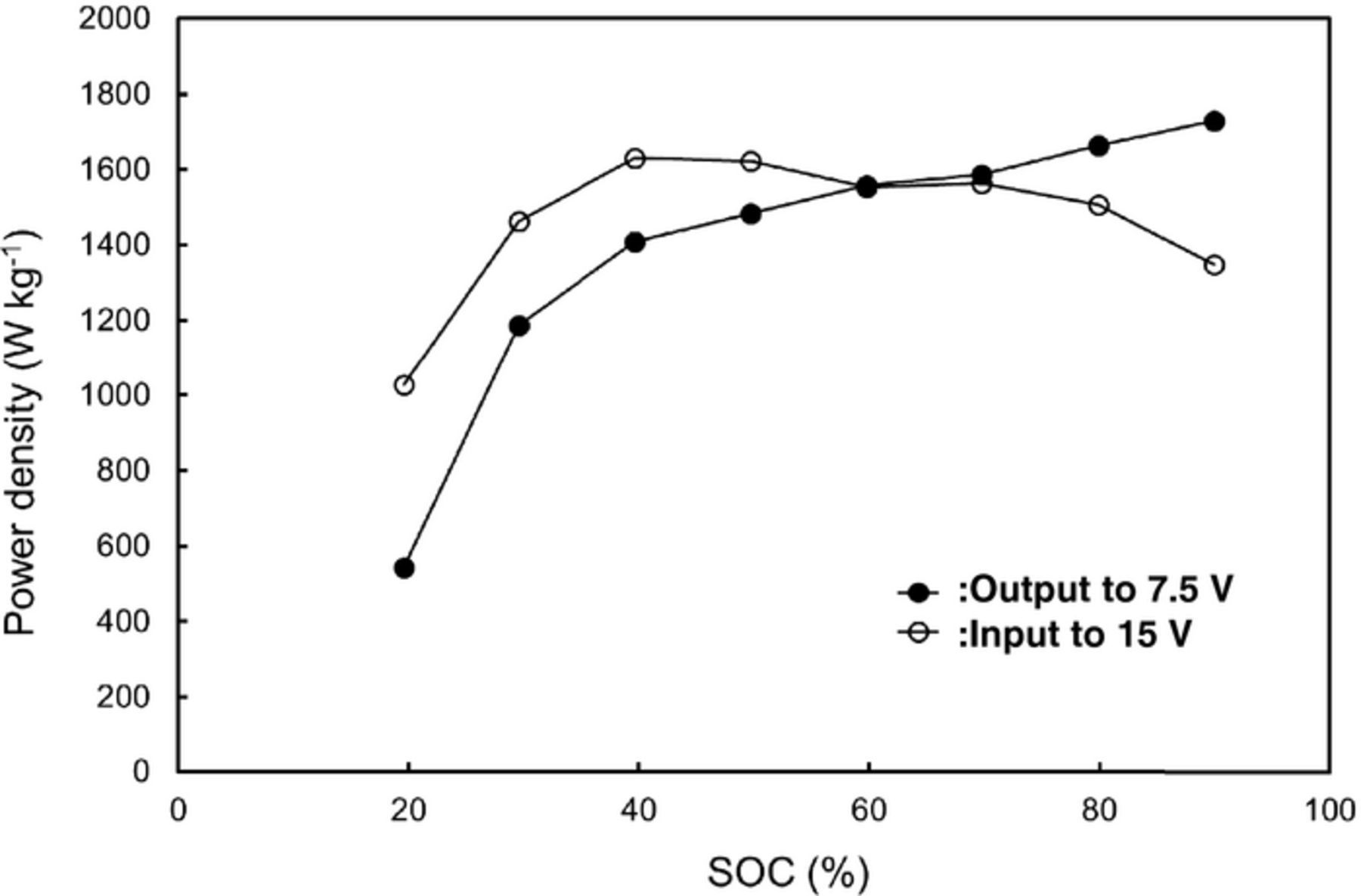
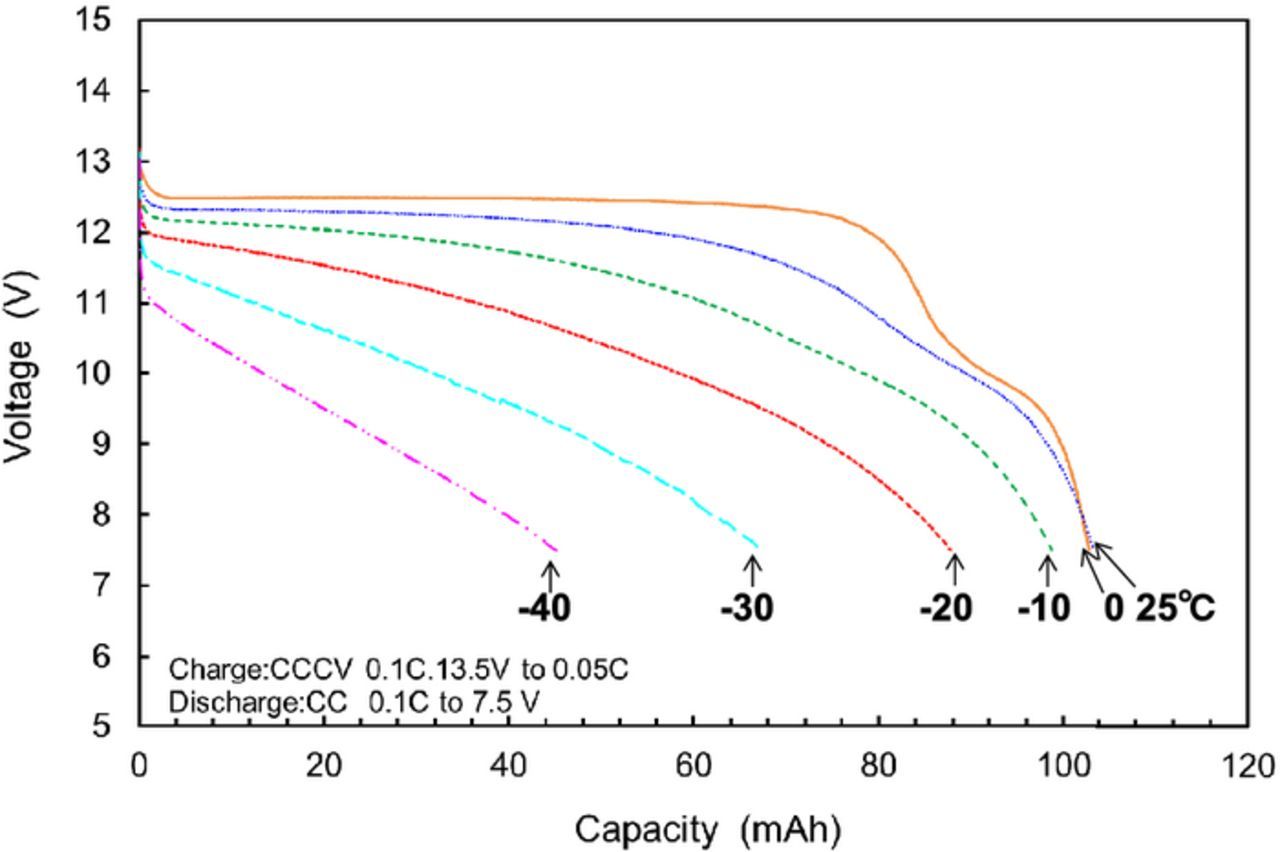
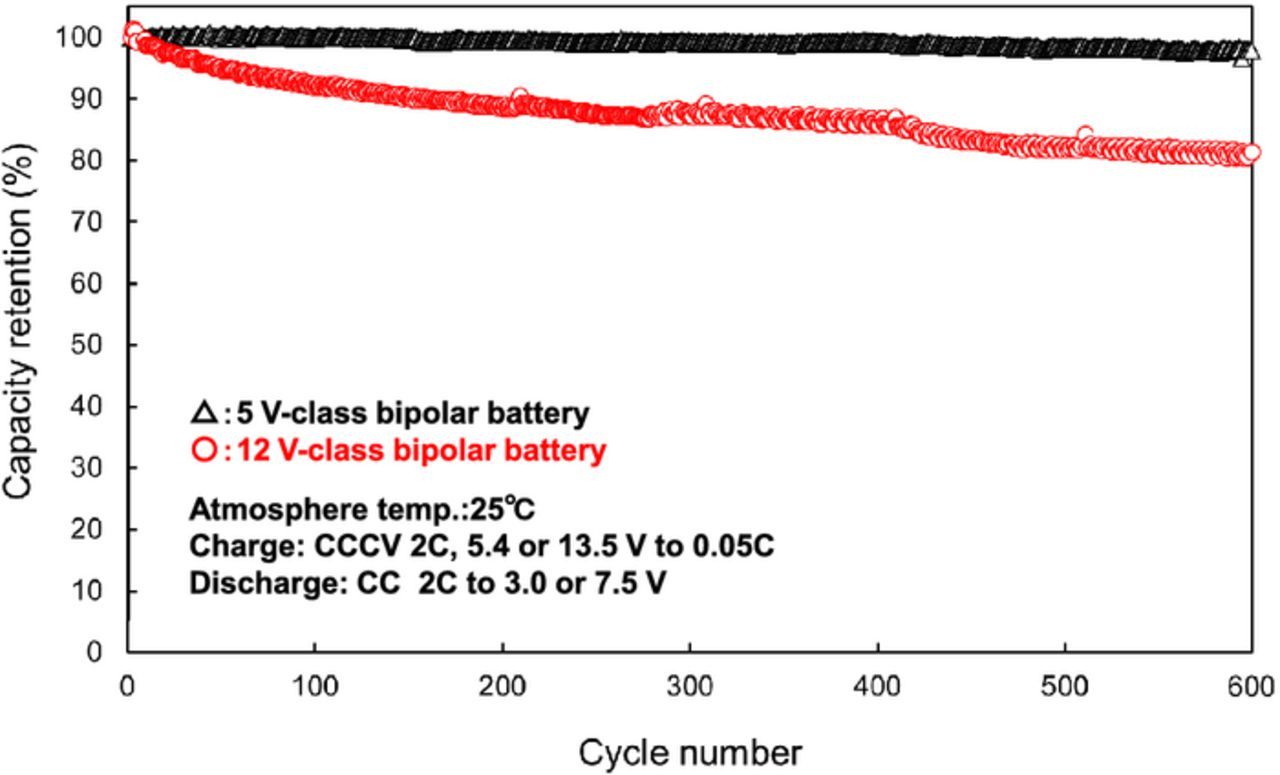
We performed a preliminary fabrication of a small-size 12 V-class bipolar LTO-based battery using LiMn0.8Fe0.2PO4 cathode and the LLZ-based hybrid solid electrolyte.10 We have been developing large-size bipolar batteries and enhanced the performance by improvements of the electrode materials. Table I summaries the specification of the fabricated 12 V-class bipolar LTO/LMFP battery. The bipolar LTO/LMFP battery with a discharge capacity of 102 mAh had a high output power density of 1500 W kg−1 for 10 s and a high energy density of 90 Wh kg−1. The power density and the energy density of the battery module are lower than those of the single battery. If large-scale 12 V-class bipolar LTO/LMFP batteries with the capacity of 10 Ah were fabricated, it is calculated that the energy density and the power density would be up to 100 Wh kg−1 and 1700 W kg−1, respectively, which values are competitive with those of a high-power lithium-ion battery module. Typical charge-discharge curve of the 12 V-class bipolar LTO/LMFP battery exhibited 12.5 V output and the usable discharge capacity of 90% at 0.2 C rate between 10 and 14 V as shown in Figure 9, indicating that the voltage behavior is compatible with the usable voltage range of 10 to 14 V of a 12 V lead-acid battery. Figure 10 shows discharge rate performance of the 12 V-class bipolar battery. The capacity retention and the average voltage at high-rate discharge of 10 C were 60% and 11 V, respectively. The discharge rate capability of the bipolar battery was almost the same as that of the single cell in Figure 7. The internal resistance of bipolar battery was significantly reduced by using the thin hybrid electrolyte without the film separator and the same Al current collector. Power capability for 10 sec of the bipolar battery measured by HPPC tests is shown in Figure 11. The output power density at 50% was 1500 W kg−1, which is comparable to high-power type battery modules. The power density maintained more than 1200 W kg−1 in the SOC range of more than 30%, which is acceptable for vehicle applications. Figure 12 shows low-temperature performance of the bipolar battery. In spite of using the LLZ solid electrolyte and the LMFP cathode, the bipolar LTO/LMFP battery was operated at a low-temperature of −40°C. Influence of the number of series-connected bipolar electrodes on cycle life was investigated from the comparison of 5 V-class and 12 V-class bipolar batteries because an increase in the numbers may cause the capacity fading to accelerate as the cycle number proceeds. Figure 13 shows cycle-life performance of 5 V-class and 12 V-class bipolar batteries at 25°C. The 5 V-class bipolar battery exhibited long cycle-life performance with the capacity retention of 98% at 600 cycles, which is comparable to that of the single LTO/LMFP cell. The 12 V-class bipolar battery exhibited the capacity retention of 82% at 600 cycles and a large capacity fading during early cycle numbers. The cycle capability of the 12 V-class bipolar battery was not yet the same as that of the 5 V-class one. For high-voltage bipolar batteries with a number of series-connections, we consider that highly accurate fabrication and the minimum difference among the electrode capacities are required in order to suppress the over-charge and the over-discharge reactions during cycles. We have been further improving the cycle-life performance of bipolar batteries in order to approximate it to that of a single cell. The cycle-life performance of the 12 V-class bipolar LTO/LMFP battery will be acceptable for lead-acid replacement batteries.
Table I. Specification of 12 V-class bipolar LTO/LMFP battery.
| 12 V-class bipolar | |
|---|---|
| Battery | LTO/LMFP battery |
| Case | Laminated film |
| LMFP cathode | LiMn0.85Fe0.1Mg0.05PO4 (89wt%) |
| LTO anode | Li4Ti5O12 (94wt%) |
| Electrolyte layer | LLZ with 4wt% PAN gel polymer |
| Thickness of electrolyte layer (μm) | 3--8 |
| Dimension (mm) | 70 × 110 × 1.8 |
| Weight (g) | 14 |
| Nominal capacity (mAh) | 102 |
| Nominal voltage (V) | 12.5 |
| Energy density (Wh/kg) | 90 |
| 10 s power density at 50%SOC (W/kg) | 1500 |
Figure 9. Typical charge-discharge curve of 12 V bipolar LTO/LMFP battery at 0.2 C and 25°C.
Figure 10. Discharge rate performance of 12 V bipolar LTO/LMFP battery at 25°C.
Figure 11. Power capability for 10 sec of 12 V bipolar LTO/LMFP battery at 25°C.
Figure 12. Low-temperature discharge performance of 12 V bipolar LTO/LMFP battery at 0.1 C rate.
Figure 13. Cycle performance of 5 V-class and 12 V-class bipolar LTO/LMFP batteries at 2C and 25°C.
Conclusions
Electrochemical properties and performance of LMFP cathode and LTO/LMFP cells were investigated in order to develop bipolar batteries using thin LLZ-based hybrid solid electrolyte for low-voltage system applications such as start-stop systems and 12 V lead-acid replacement batteries. Five series-connected LTO/LMFP cells exhibited good harmonization with the useable voltage range of a 12 V lead-acid battery. The LTO/LMFP cell exhibited excellent high-temperature cycle life performance with the capacity retention of 92% at 1800 cycles and 60°C compared with LTO/LMO cell, which was attributed to very small Mn dissolution from the LMFP cathode. Durability against over-discharge cycling of the LMFP cathode was significantly superior to other 4 V-class cathodes. Cyclic voltammograms of the LTO/Al and LMFP/Al electrodes in the LLZ-based hybrid solid electrolyte indicated the electrochemical stability of both electrodes without the irreversible reactions in the potential range between 1 and 4.5 V vs. Li/Li+. These results indicate that the bipolar electrode consisting of the LTO anode and the LMFP cathode on the same Al current collector is a promising choice for 12 V battery applications. The LTO/LMFP cells using the thin hybrid solid electrolyte exhibited not only high-rate discharge but also a low self-discharge for practical use. We fabricated the 12 V-class bipolar LTO/LMFP battery with the discharge capacity of 102 mAh. The discharge voltage of 12.5 V at 0.2 C rate, the energy density of 90 Wh kg−1, and the output power density of 1500 W kg−1 for 10 s were obtained, which performances are superior to those of conventional high-power battery modules for hybrid electric vehicles and start-stop system applications. Comparison of cycle-life performance between 5 V-class and 12 V-class bipolar batteries indicated that high-voltage bipolar batteries with a number of series-connections require highly accurate fabrication and the minimum difference among the electrode capacities to obtain long cycle-life. The liquid-free and separator-free 12 V-class bipolar LTO/LMFP battery is expected to be suitable for low-voltage system applications because of light weight, high-power, long-life, low costs, and safety.