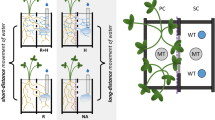
The role of mycorrhizae in plant water relations has been controversial since it was proposed over a century ago. Improvements in plant water throughput and associated carbon fixation by mycorrhizal plants have been demonstrated since the early 1970s, but the mechanisms remain poorly understood. Mechanism studies have concentrated in greenhouse pot studies. These studies are limited because roots readily become pot-bound, in contrast to the distinct spatial stratification in the field. Water transport studies also focused on symplastic water flow rates. Recent data from hyphal structure and using models suggest that apoplastic transport may be the focal mechanism under drought conditions. Hydraulic redistribution along mycorrhizal fungal hyphae indicates that apoplastic transport and water potential gradients, especially in arid lands, regulate water flow patterns along hyphae. New sensor technologies may open the possibility of greater study of water and nutrient dynamics in situ where these mechanisms can be studied in detail.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Alexopoulos CJ, Mims CW, Blackwell M (1996) Introductory mycology, 4th edition. Wiley, New York
Allen MF (1980) Physiological alterations associated with vesicular-arbuscular mycorrhizal infection of Bouteloua gracilis. Ph.D. Dissertation, University of Wyoming, Laramie
Allen MF (1982) Influence of vesicular-arbuscular mycorrhizae on water movement through Bouteloua gracilis. New Phytol 91: 191–196
Allen MF (1987) Reestablishment of mycorrhizae on Mount St. Helens: migration vectors. Trans Br Mycol Soc 88: 413–417
Allen MF (1996). The ecology of arbuscular mycorrhizae: A look back into the 20th century and a peek into the 21st centenary review article, British mycological society. Mycol Res 100: 769–782
Allen MF (2006). Water dynamics of mycorrhizas in arid soils. In: Gadd GM (ed) Fungi in biogeochemical cycles. Cambridge University Press, New York, pp 74–97
Allen MF (2007) Mycorrhizal fungi: highways for water and nutrients in arid soils. Vadose Zone 6: 291–297
Allen EB, Allen MF (1986) Water relations of xeric grasses in the field: interactions of mycorrhizas and competition. New Phytol 104: 559–571
Allen MF, Boosalis MG (1983) Effects of two species of vesicular-arbuscular mycorrhizal fungi on drought tolerance of winter wheat. New Phytol 93: 67–76
Allen EB, Cunningham GL (1983) Effects of vesicular-arbuscular mycorrhizae on Distichlis spicata under three salinity levels. New Phytol 93: 227–236
Allen MF, Moore TS Jr, Christensen M (1980) Phytohormone changes in Bouteloua gracilis infected by vesicular-arbuscular mycorrhizae: I. Cytokinin increases in the host plant. Can J Bot 58: 371–374
Allen MF, Smith WK, Moore TS Jr, Christensen M (1981) Comparative water relations and photosynthesis of mycorrhizal and nonmycorrhizal Bouteloua gracilis H.B.K. Lag ex Steud. New Phytol 88: 683–693
Allen MF, Moore TS Jr, Christensen M (1982) Phytohormone changes in Bouteloua gracilis infected by vesicular-arbuscular mycorrhizae: II. Altered levels of gibberellin-like substances and abscisic acid in the host plant. Can J Bot 60: 468–471
Allen MF, Allen EB, Stahl PD (1984) Differential niche response of Bouteloua gracilis and Pascopyrum smithii to VA mycorrhizae. Bull Torrey Bot Club 111: 361–365
Allen MF, Crisafulli C, Friese CF, Jeakins SL (1992) Re-formation of mycorrhizal symbioses on Mount St. Helens, 1980–1990: Interactions of rodents and mycorrhizal fungi. Mycol Res 98: 447–453
Allen MF, Lansing J, Allen EB (2002) The role of mycorrhizal fungi in composition and dynamics of plant communities: A scaling issue. Prog Bot 63: 344–367
Allen MF, Swenson W, Querejeta JL, Egerton-Warburton LM, Treseder KK (2003). Ecology of mycorrhizae: A conceptual framework for complex interactions among plants and fungi. Annu Rev Phytopath 41: 271–303
Allen MF, Vargas R, Graham EA, Swenson W, Hamilton M, Taggart M, Harmon TC, Tatko A, Rundel P, Fulkerson B, Estrin D (2007) Soil sensor technology: Life within a pixel. BioScience 57: 859–867
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11: 3–42
Augé RM, Schekel KA, Wample RL (1986) Greater leaf conductance of well watered VA mycorrhizal plants is not related to phosphorus nutrition. New Phytol 103: 107–116
Augé RM, Sylvia DM, Park S, Buttery BR, Saxton AM, Moore JL, Cho K (2004) Partitioning mycorrhizal influence on water relations of Phaseolus vulgaris into soil and plant components. Can J Bot 82: 503–514
Azcón-Aguilar C, Barea JM (1997) Mycorrhizal dependency of a representative plant species in Mediterranean shrublands (Lavandula spica L.) as a key factor to its use for revegetation strategies in desertification-threatened areas. Appl Soil Ecol 7: 83–92
Bago B, Azcon-Aguilar C, Piche Y (1998) Architecture and developmental dynamics of the external mycelium of the arbuscular mycorrhizal fungus Glomus intraradices grown under monoxenic conditions. Mycologia 90: 52–62
Bornyasz MA, Graham RC, Allen MF (2005). Ectomycorrhizae in a soil-weathered granitic bedrock regolith: linking matrix resources to plants. Geoderma 126: 141–160
Davies FT, Olalde-Portugal V, Aguilera-Gomez L, Alvarado MJ, Ferrera-Cerrato RC, Boutton TW (2002) Alleviation of drought stress of Chile ancho pepper (Capsicum annuum L. cv. San Luis) with arbuscular mycorrhiza indigenous to Mexico. Scientia Horticulturae 92: 347–359
Di JJ, Allen EB (1991) Physiological responses of six wheatgrass cultivars to mycorrhizae. J Range Manage 44: 336–341
Duddridge JA, Malibari A, Read, DJ (1980) Structure and function of mycorrhizal rhizomorphs with special reference to their role in water transport. Nature 287: 834–836
Egerton-Warburton LM, Graham RC, Hubbert KR (2003). Spatial variability in mycorrhizal hyphae and nutrient and water availability in a soil-weathered bedrock profile. Plant Soil 249: 331–342
Egerton-Warburton LM, Querejeta JI, Allen, MF (2007). Common mycorrhizal networks provide a potential pathway for the transfer of hydraulically lifted water between plants. J Exp Bot 58: 1473–1483
Egerton-Warburton LM, Querejeta JI, Allen MF (in press) Efflux of hydraulically lifted water from mycorrhizal fungal hyphae during imposed drought. Plant Signal Behav
Friese CF, Allen MF (1991) The spread of VA mycorrhizal fungal hyphae in the soil: inoculum types and external hyphal architecture. Mycologia 83: 409–418
Gerdemann JW (1968) Vesicular-arbuscular mycorrhiza and plant growth. Annu Rev Phytopath 6: 397–418
Hamilton MP, Graham EA, Rundel PW, Allen MF, Kaiser W, Hansen MH, Estrin DL (2007) New approaches in embedded networked sensing for terrestrial ecological observatories. Environ Eng Sci 24: 192–204
Hardie K (1985) The effect of removal of extraradical hyphae on water uptake by vesicular-arbuscular mycorrhizal plants. New Phytol 101: 677–684
Hardie K, Leyton L (1981) The influence of vesicular-arbuscular mycorrhiza on growth and water relations of red-clover. 1. In phosphate deficient soil. New Phytol 89: 599–608
Hatch AB (1937) The physical basis of mycotrophy in Pinus. Black Rock Forest Bull 6: 1–168
Hickson LD (1993) The effects of vesicular-arbuscular mycorrhizal fungi on the light harvesting, gas exchange, and architecture of sagebrush (Artemisia tridentata. ssp. tridentata). M.S. Thesis, San Diego State University, San Diego, CA
Hobbie EA, Colpaert JV (2004) Nitrogen availability and mycorrhizal colonization influence water use efficiency and carbon isotope patterns in Pinus sylvestris. New Phytol 164: 515–525
Jury WA, Gardner WR, Gardner WH (1991) Soil physics, 5th edition. Wiley, New York
Khalvati MA, Hu Y, Mozafar A, Schmidhalter U (2005) Quantification of water uptake by arbuscular-mycorrhizal hyphae and its significance for leaf growth, water relations, and gas exchange of barley subjected to drought stress. Plant Biol 7: 706–712
Kucey RMN, Paul EA (1982) Carbon flow, photosynthesis, and N2 fixation in mycorrhizal and nodulated faba beans (Vicia faba L.). Soil Biol Biochem 14: 407–412
Marks GC, Kozlowski TT (1973) Ectomycorrhizae; their structure and function. Academic, New York
Mena-Violante HG, Ocampo-Jimenez O, Dendooven L, Martinez-Soto G, Gonzalez-Castaneda J, Davis FT, Olalde-Portugal V (2006) Arbuscular mycorrhizal fungi enhance fruit growth and quality of chile ancho (Capsicum annuum L. cv San Luis) plants exposed to drought. Mycorrhiza 16: 261–267
Mexal J, Reid CPP (1973) The growth of selected mycorrhizal fungi in response to induced water stress. Can J Bot 51: 1579–1588
Mosse B (1973) Advances in study of arbuscular-arbuscular mycorrhiza. Annu Rev Phytopath 11: 171–196
Nobel PS (1974) Biophysical plant physiology. Freeman Press, New York
Querejeta JI, Barea JM, Allen MF, Caravaca F, Roldan A (2003a) Differential response of δ13C and water use efficiency to arbuscular mycorrhizal infection in two aridland woody plant species. Oecologia 135: 510–515
Querejeta JI, Egerton-Warburton LM, Allen MF (2003b) Direct nocturnal water transfer from oaks to their mycorrhizal symbionts during severe soil drying. Oecologia 134: 55–64
Querejeta JI, Allen MF, Caravaca F, Roldan A (2006). Differential modulation of host plant δ13C and δ18O by native and nonnative arbuscular mycorrhizal fungi in a semiarid environment. New Phytol 169: 379–387
Querejeta JI, Allen MF, Alguacil MM, Roldan A (2007a) Plant isotopic composition provides insight into mechanisms underlying growth stimulation by AM fungi in a semiarid environment. Funct Plant Biol 34: 683–691
Querejeta JI, Egerton-Warburton LM, Allen MF (2007b) Hydraulic lift may buffer rhizosphere hyphae against the negative effects of severe soil drying in a California oak savanna. Soil Biol Biochem 39: 409–417
Reid CPP, Woods FW (1969) Translocation of C14-labeled compounds in mycorrhizae and its implications in interplant nutrient cycling. Ecology 50: 179–181
Rillig MC, Mummey DL (2006) Mycorrhizas and soil structure. New Phytol 171: 41–53
Ruiz-Lozano JM, Porcel R, Aroca R (2006) Does the enhanced tolerance of arbuscular mycorrhizal plants to water deficit involve modulation of drought-induced genes? New Phytol 171: 693–698
Safir GR, Boyer JS, Gerdemann JW (1971) Mycorrhizal enhancement of water transport in soybeans. Science 172: 581–583
Safir GR, Boyer JS, Gerdemann JW (1972) Nutrient status and mycorrhizal enhancement of water transport in soybeans. Plant Physiol 49: 700–703
Stahl E (1900). Der Sinn der Mycorrhizenbildung. Jahrbücher für wissenschaftliche Botanik 34: 539–668
Stolp H (1988) Microbial ecology: Organisms, habitats, activities. Cambridge University Press, New York
Valentine AJ, Mortimer PE, Lintnaar A, Borgo R (2006) Drought responses of arbuscular mycorrhizal grapevines. Symbiosis 41: 127–133
Vargas R, Allen MF, Allen EB (2008) Biomass and carbon accumulation in a fire chronosequence of a seasonally dry tropical forest. Global Change Biol 14: 109–124
Warrick AW (2003) Soil water dynamics. Oxford University Press, New York
Wu QS, Xia RX (2006) Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J Plant Physiol 163: 417–425
Author information
Authors and Affiliations
Corresponding author
Editor information
Rights and permissions
Copyright information
© 2009 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Allen, M.F. (2009). Water Relations in the Mycorrhizosphere. In: Lüttge, U., Beyschlag, W., Büdel, B., Francis, D. (eds) Progress in Botany. Progress in Botany, vol 70. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-68421-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-540-68421-3_12
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-68420-6
Online ISBN: 978-3-540-68421-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)