Summary
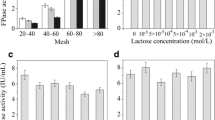
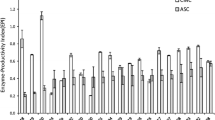
The enzyme loading needed to achieve substrate saturation appeared to be the most economical enzyme concentration to use for hydrolysis, based on percentage hydrolysis. Saturation was reached at 25 filter paper units per gram substrate on Solka Floc BW300, as determined by studying (a) initial adsorption of the cellulase preparation onto the substrate, (b) an actual hydrolysis or (c) a combined hydrolysis and fermentation (CHF) process. Initial adsorption of the cellulases onto the substrate can be used to determine the minimal cellulase requirements for efficient hydrolysis since enzymes initially adsorbed to the substrate have a strong role in governing the overall reaction. Trichoderma harzianum E58 produces high levels of β-glucosidase and is able to cause high conversion of Solka Floc BW300 to glucose without the need for exogenous β-glucosidase. End-product inhibition of the cellulase and β-glucosidase can be more effectively reduced by employing a CHF process than by supplemental β-glucosidase.
Similar content being viewed by others
References
Blotkamp PJ, Takagi M, Pemberton MS, Emert GH (1978) Enzymatic hydrolysis of cellulose and simultaneous fermentation to alcohol. Am Inst Chem Eng Symp Ser no. 181 74:85–90
Frennesson I, Tragardh G, Hahn-Hagerdal B (1985) An ultrafiltration membrane reactor for obtaining experimental reaction rates at defined concentrations of inhibiting sugars during enzymatic saccharification of alkali-pretreated sallow:formulation of a simple empirical rate equation. Biotechnol Bioeng 27:1328–1334
Gilbert IG, Tsao GT (1985) Surface adsorption and reaction kinetics of enzymatic cellulose hydrolysis in a column reactor. Annu Rep Ferment Process 8:211–297
Hogan CM (1989) Enzymatic and structural factors limiting hydrolysis of cellulose by Trichoderma harzianum E58 cellulases. MSc Thesis. University of Ottawa
Kyriacou A, Neufeld RJ, MacKenzie CR (1988) Effect of physical parameters on the adsorption characteristics of fractionated Trichoderma reesei cellulase components. Enzyme Microb Technol 10:675–681
Ladisch MR, Lin KW, Voloch M, Tsao GT (1983) Process considerations in the enzymatic hydrolysis of biomass. Enzyme Microb Technol 5:82–102
Lee YH, Fan LT (1983) Kinetic studies of enzymatic hydrolysis of insoluble cellulose: (II) analysis of extended hydrolysis times. Biotechnol Bioeng 25:939–966
Lee SB, Shin HS, Ryu DDY, Mandels M (1982) Adsorption of cellulase on cellulose: effect of physiocochemical properties of cellulose on adsorption and rate of hydrolysis. Biotechnol Bioeng 24:2137–2153
Lee SB, Kim IH, Ryu DDY, Taguchi H (1983) Structural properties of cellulose and cellulase reaction mechanism. Biotechnol Bioeng 25:33–51
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mandels M, Andreotti R, Roche C (1976) Measurement of saccharifying cellulase. Biotechnol Bioeng Symp 6:21–33
Mes-Hartree M, Hogan CM, Saddler JN (1988) Influence of growth substrate on production of cellulase enzymes by Trichoderma harzianum E58. Biotechnol Bioeng 31:725–729
Miller GL (1959) Use of dinitrosalicylic reagent for the determination of reducing sugars. Anal Chem 31:426–428
Mukataka S, Tada M, Takahashi J (1983) Effects of agitation on enzymatic hydrolysis of cellulose in a stirred-tank reactor. J Ferment Technol 61:615–621
Ooshima H, Ishitani Y, Harano Y (1985) Simultaneous saccharification and fermentation of cellulose: effect of ethanol on enzymatic saccharification of cellulose. Biotechnol Bioeng 27:389–397
Peitersen N, Medeiros J, Mandels M (1977) Adsorption of Trichoderma cellulase on cellulose. Biotechnol Bioeng 19:1091–1094
Raabo E, Terkildsen TC (1960) On the determination of blood glucose. Scand J Clin Lab Invest 12:402–406
Rainbow C (1970) Brewer's yeasts. In: Rose AH, Harrison JS (eds) The yeasts, vol 3. Academic Press, New York, pp 147–224
Ryu DDY, Lee SB, Tassinari T, Macy C (1982) Effect of compression milling on cellulose structure and on enzymatic hydrolysis kinetics. Biotechnol Bioeng 24:1047–1067
Saddler JN, Hogan C, Chan MKH, Louis-Seize G (1982) Ethanol fermentation of enzymatically hydrolysed pretreated wood fractions using Trichoderma cellulases, Zymomonas mobilis and Saccharomyces cerevisiae. Can J Microbiol 23:1311–1319
Saddler JN, Hogan CM, Louis-Seize G (1985) A comparison between the cellulase systems of Trichoderma harzianum E58 and Trichoderma reesei C30. Appl Microbiol Biotechnol 22:139–145
Tan LUL, Yu EKC, Mayers P, Saddler JN (1986) Column cellulose hydrolysis reactor: cellulase adsorption profile. Appl Microbiol Biotechnol 25:256–261
Wald S, Wilke CR, Blanch HW (1984) Kinetics of the enzymatic hydrolysis of cellulose. Biotechnol Bioeng 26:221–230
Wilke CR, Yang RD, Sciamanna AF, Freitas RP (1981) Raw materials evaluation and process development studies for conversion of biomass to sugars and ethanol. Biotechnol Bioeng 23:163–183
Yu EKC, Saddler JN (1982) Enhanced production of 2,3-butanediol by Klebsiella pneumoniae grown on high sugar concentration in the presence of acetic acid. Appl Environ Microbiol 44:777–784
Author information
Authors and Affiliations
Additional information
Offprint requests to: C. M. Hogan
Rights and permissions
About this article
Cite this article
Hogan, C.M., Mes-Hartree, M., Saddler, J.N. et al. Assessment of methods to determine minimal cellulase concentrations for efficient hydrolysis of cellulose. Appl Microbiol Biotechnol 32, 614–620 (1990). https://doi.org/10.1007/BF00173736
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00173736