Abstract
Lithium salts of di-n-pentyl (DPP),n-butyl(n-hexyl) (BHP),n-propyl(n-hexyl) (PHP) and ethyl(n-octyl) (EOP) phosphates were synthesized and the phase diagrams of the lithium phosphate-water binary systems were determined. The phase diagrams of the DPP-, BHP- and PHP-water systems contain three regions (I, II and III) in common, which correspond to a homogeneous transparent one-phase solution, and lyotropic liquid crystalline and coagel phases, respectively. However, the EOP-H2O system contains an additional hard gel phase (region IV).
31P NMR spectra suggest that region I is a monomer⇔micelle equilibrium phase and region II is a lamellar phase. X-ray diffraction results show that for the DPP-, BHP-and PHP-water systems the twon-alkyl chains are closely packed in the lamellar phase in a manner which alternatively combines short and long chains, while in EOP-water system the two long chains are loosely packed. Furthermore, it may be assumed from31P NMR spectra and x-ray diffraction results that region IV in the EOP-water system is a cubic phase.
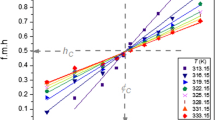
Thermotropic properties for these DAP-water systems were also investigated by DSC temperature profile curves. From the ΔH variation upon the II→I thermal transition, we assumed that stability of the aggregate structure in the liquid crystalline state increases in the order EOP<PHP<BHP<DPP. Thus, we have found that thermotropic properties for a series of DAP-water binary system are closely correlated with the extent of asymmetric molecular shape in DAP.
Similar content being viewed by others
References
Kunitake T, Okahata Y (1977) J Amer Chem Soc 99:3860–3862
Fendler JH (1980) Acc Chem Res 13:7–13
Fuhrhop JH, Mathieu J (1984) Angew Chem 96:124–137
Sudhölter EJR, Engberts JBFN, Hoekstra D (1980) J Amer Chem Soc 102:2467–2469
Sudhölter EJR, De Grip WJ, Engberts JBFN (1982) J Amer Chem Soc 104:1069–1072
Kano K, Romero A, Djermouni B, Acke HJ, Fendler JH (1979) J Amer Chem Soc 101:4030–4037
Kumano A, Kajiyama T, Takayanagi M, Kunitake T, Okahata Y (1984) Ber Bunsen-Ges Phys Chem 88:1216–1222
Shimomura M, Kunitake T (1982) J Amer Chem Soc 104:1757–1759
Murakami Y, Nakano A, Hoshimatsu A, Uchitomi K, Matsuda Y (1984) J Amer Chem Soc 106:3613–3623
Carmona-Ribeiro AM, Chaimovich H (1983) Biochim Biophys Acta 733:172–179
Carmona-Ribeiro AM, Yoshida LS, Sesso A, Chaimovich H (1984) J Colloid Interface Sci 100:433–443
Rupert LAM, Hoekstra D, Engberts JBFN (1985) J Amer Chem Soc 107:2628–2631
Rupert LAM, Engberts JBFN, Hoekstra D (1986) J Amer Chem Soc 108:3920–2925
Rupert LAM, Van Breemen JFC, Van Bruggen EFJ, Engberts JBFN, Hoekstra D (1987) J Membr Biol 95:255–263
Rupert LA, Hoekstra D, Engberts JBFN (1987) J Colloid Interface Sci 120: 125–134
Shimomura M, Kunitake T (1981) Chem Lett 1001–1004
Israelachvili JN, Mitchell DJ, Ninham BW (1976) J Chem Soc Faraday Trans I 72:1525–1568
Ekwall P (1975) Adv Liq Cryst 1:1–142
Skoulios A (1979) Am Phys 3:421–450
Vincent JM, Skoulios A (1966) Acta cryst 20:432–440, 441–446, 447–451
Puvvada S, Blanckstein D (1990) J Chem Phys 92:3710–3724
Kato T, Seimiya T (1986) J Phys Chem 90:3159–3167
Herrington TM, Sahi SS (1988) J Colloid Interface Sci 121:107–120
Missel PJ, Mazer NA, Benedek GB, Young CY, Carey MC (1980) J Phys Chem 84:1044–1057
Malliaris A, Le Moigne J, Sturm J, Zana R (1985) J Phys Chem 89:2709–2713
Carnie SL, Israelachvili JN, Pailthorpe BA (1979) Biochim Biophys Acta 554: 340–357
Zana R, Talmon Y (1993) Nature 362: 228–230
McCombie H, Saunders BC, Stacey GJ (1945) J Chem Soc 380–382
Mukaiyama T, Fujisawa T (1961) Bull Chem Soc Jpn 34:812–813
Hirata H, Katayama S, Okabayashi H, Furusaka M, Kawakatsu T (1995) Colloid Polym Sci in press
Roberts MF, Adamich M, Robson RJ, Dennis EA (1979) Biochemistry 18: 3301–3308
Matsushita K, Kamo O, Terada Y, Yoshida T, Okabayashi H (1984) Chem Scripta 23:228–232
Crutchfield MM, Callis CF, Irani RR, Roth GC (1962) Inorg Chem 1:813–817
Jardetsky O, Wertz JE (1960) J Amer Chem Soc 82:318–323
Chachaty C, Quagebeur JP (1983) J Phys Chem 87:4341–4343
Yoshida T, Miyagai K, Taga K, Okabayashi H, Matsushita K (1990) Magn Reson Chem 28:715–721
Yoshida T, Miyagai K, Aoki S, Taga K, Okabayashi H (1991) Colloid Polym Sci 269:713–719
Cullis PR, de Kruijff B (1979) Biochem Biophys Acta 559:399–420
Lawaon KD, Mabis AJ, Flautt TJ (1968) J Phys Chem 72:2058–2065
Kodama M, Kuwabara M, Seki S (1982) Biochem Biophys Acta 689:567–570
Hirata H, Ogasawara T, Okabayashi H, unpublished data to be published separately
Ulmius J, Wennerstrom H, Lindblom G, Arvidson G (1977) Biochemistry 16: 5742–5745
Okabayashi H, Taga K, Miyagai M, Uehara T, Yoshida T, Nishio E (1991) J Phys Chem 95:7932–7938
Okabayashi H, Hirata H, Suzuki Y, Taga K, Mathew C (1995) Vibrational Spectroscopy in press
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hirata, H., Maegawa, K., Kawamatsu, T. et al. Phase diagrams and phase structures of identical and mixed chain lithium di-n-alkyl phosphate-water binary systems. Asymmetric molecular shape effect. Colloid Polym Sci 274, 654–661 (1996). https://doi.org/10.1007/BF00653064
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00653064