Summary
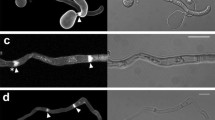
The penetration peg is the structure used byMagnaporthe grisea to pierce the surface of rice leaves or very hard nonbiodegradable substrates. Penetration pegs produced by appressoria in vitro were examined by electron microscopy and immunofluorescence microscopy using various fluorophore labeled anti-actins. Freeze-substitution preparation of appressoria at early stages of substrate penetration showed that peg cytoplasm consisted primarily of a zone of exclusion, excluding even ribosomes, and was continuous with a similar region in the appressorium. Apical vesicles were, however, observed in short, presumably elongating pegs. Immunofluorescence microscopy was used to demonstrate binding of a monoclonal anti-actin to penetration peg cytoplasm, following “permeabilization” of appressoria by means of a brief sonication. Occasional filaments and ca. 300 nm diameter plaques were labeled in appressorial cytoplasm. Western blot analysis of germ tube extracts showed that the monoclonal probe bound predominantly to a single band with a molecular weight similar to that of rabbit muscle actin. Preincubation of the antibody with actin virtually eliminated peg labeling. We conclude that the penetration peg contains actin which may play a role in the formation of the zone of exclusion.
Similar content being viewed by others
Abbreviations
- PE:
-
polyethylene
- Tris:
-
tris(hydroxymethyl)-amino-methane
- TBS:
-
Tris-buffered saline
- TBS-B:
-
Tris buffered saline plus 3% bovine serum albumin
References
Adams AEM, Pringle JR (1984) Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutantSaccharomyces cerevisiae. J Cell Biol 98: 934–945
Anderson JM, Soll DR (1986) Differences in actin localization during bud and hypha formation in the yeastCandida albicans. J Gen Microbiol 132: 2035–2047
Bourett TM, Howard RJ (1990) In vitro development of penetration structures in the rice blast fungusMagnaporthe grisea. Can J Bot 68: 329–342
— — (1991) Ultrastructural immunolocalization of actin in a fungus. Protoplasma 163: 199–202
Bray D, Heath J, Moss D (1986) The membrane-associated “cortex” of animal cells: its structure and mechanical properties. J Cell Sci [Suppl] 4: 71–88
Butt TM, Heath IB (1988) The changing distribution of actin and nuclear behavior during the cell cycle of the mite-pathogenic fungusNeozygites sp. Eur J Cell Biol 46: 499–505
Crawford MS, Chumley FG, Weaver CG, Valent B (1986) Characterization of the heterokaryotic and vegetative diploid phases ofMagnaporthe grisea. Genetics 114: 1111–1129
Drenckhahn D, Engel K, Höfer D, Merte C, Tilney L, Tilney M (1991) Three different actin filament assemblies occur in every hair cell: each contains a specific actin crosslinking protein. J Cell Biol 112: 641–651
Gegenheimer P (1990) Preparation of extracts from plants. In: Deutscher MP (ed) Guide to protein purification. Academic Press, San Diego, pp 174–193 (Methods in enzymology, vol 182)
Giloh H, Sedat JW (1982) Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates byn-propyl gallate. Science 217: 1252–1255
Girbardt M (1979) A microfilamentous septal belt (FSB) during induction of cytokinesis inTrametes versicolor (L. ex Fr.). Exp Mycol 3: 215–228
Hasek J, Rupes I, Svobodova J, Streiblova E (1987) Tubulin and actin topology during zygote formation ofSaccharomyces cerevisiae. J Gen Microbipl 133: 3355–3363
Heath IB (1987) Preservation of a labile cortical array of actin filaments in growing hyphal tips of the fungusSaprolegnia ferax. Eur J Cell Biol 44: 10–16
— (1990) The roles of actin in tip growth of fungi. Int Rev Cytol 123: 95–127
Hoch HC, Howard RJ (1980) Ultrastructure of freeze-substituted hyphae of the basidiomyceteLaetisaria arvalis. Protoplasma 103: 281–297
—, Staples RC (1983 a) Visualization of actin in situ by rhodamineconjugated phalloin in the fungusUromyces phaseoli. Eur J Cell Biol 32: 52–58
— — (1983b) Ultrastructural organization of the non-differentiated uredospore germling ofUromyces phaseoli varietytypica. Mycologia 75: 795–824
Howard RJ (1981) Ultrastructural analysis of hyphal tip cell growth in fungi: Spitzenkörper, cytoskeleton and endomembranes after freeze-substitution. J Cell Sci 48; 89–103
—, Ferrari MA (1989) Role of melanin in appressorium function. Exp Mycol 13: 403–418
—, O'Donnell KL (1987) Freeze substitution of fungi for cytological analysis. Exp Mycol 11: 250–269
—, Ferrari MA, Roach DH, Money NP (1991) Penetration of hard substrates by a fungus employing enormous turgor pressures. Proc Natl Acad Sci USA 88: 281–284
Jackson SL, Heath IB (1990) Visualization of actin arrays in growing hyphae of the fungusSaprolegniaferax. Protoplasma 154: 66–70
Kanbe T, Kobayashi I, Tanaka K (1989) Dynamics of cytoplasmic organelles in the cell cycle of the fission yeastSchizosaccharomyces pombe: three-dimensional reconstruction from serial sections. J Cell Sci 94: 647–656
Kilmartin JV, Adams AEM (1984) Structural rearrangements of tubulin and actin during the cell cycle of the yeastSaccharomyces. J Cell Biol 98: 922–933
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T 4. Nature 227: 680–685
Lessard JL (1988) Two monoclonal antibodies to actin: one muscle selective and one generally reactive. Cell Motil Cytoskeleton 10: 349–362
Marks J, Hyams JS (1985) Localization of F-actin through the cell division cycle ofSchizosaccharomyces pombe. Eur J Cell Biol 39: 27–32
Newhouse JR, Hoch HC, MacDonald WL (1983) The ultrastructure ofEndothia parasitica. Comparison of a virulent with a hypovirulent isolate. Can J Bot 61: 389–399
O'Keefe DP, Leto KJ (1989) Cytochrome P-450 from the mesocarp of avocado (Persea americana). Plant Physiol 89: 1141–1149
Pardee JD, Spudich JA (1982) Purification of muscle actin. Methods Enzymol 85: 164–181
Patton AM, Marchant R (1978) An ultrastructural study of septal development in hyphae ofPolyporus biennis. Arch Microbiol 118: 271–277
Picton JM, Steer MW (1985) A model for the mechanism of tip extension in pollen tubes. J Theor Biol 98: 15–20
Raudaskoski M, Salo V, Mini SS (1988) Structure and function of the cytoskeleton in filamentous fungi. Karstenia 28: 49–60
—, Rupes I, Timonen S (1991) Immunofluorescence microscopy of the cytoskeleton in filamentous fungi after quick-freezing and low-temperature fixation. Exp Mycol 15: 167–173
Roberson RW (1992) The actin cytoskeleton in hyphal cells ofSclerotium rolfsii. Mycologia 84 (in press)
—, Fuller MS (1988) Ultrastructural aspects of the hyphal tip ofSclerotium rolfsii preserved by freeze substitution. Protoplasma 146: 143–149
Rossman AY, Howard RJ, Valent B (1990)Pyricularia grisea, the correct name for the rice blast disease fungus. Mycologia 82: 509–512
Runeberg P, Raudaskoski M, Virtanen I (1986) Cytoskeletal elements in the hyphae of the homobasidiomyceteSchizophyllum commune visualized with indirect immunofluorescence and NBD-phallacidin. Eur J Cell Biol 41: 25–32
Salo V, Niini SS, Virtanen I, Raudaskoski M (1989) Comparative immunocytochemistry of the cytoskeleton in filamentous fungi with dikaryotic and multinucleate hyphae. J Cell Sci 94: 11–24
Soll DR, Mitchell LH (1983) Filament ring formation in the dimorphic yeastCandida albicans. J Cell Biol 96: 486–493
Tang X, Lancelle SA, Hepler PK (1989) Fluorescence microscopic localization of actin in pollen tubes: comparison of actin antibody and phalloidin staining. Cell Motil Cytoskeleton 12: 216–224
Temperli E, Roos U-P, Hohl HR (1990) Actin and tubulin cytoskeletons in germlings of the oomycete fungusPhytophthora infestans. Eur J Cell Biol 53: 75–88
Tiburzy R, Hoch HC, Staples RC (1990) Isolation and identification of actin from the phytopathogenic filamentous fungusUromyces appendiculatus. Eur J Cell Biol 53: 364–372
Tucker BE, Hoch HC, Staples RC (1986) The involvement of Factin inUromyces cell differentiation: the effects of cytochalasin E and phalloidin. Protoplasma 135: 88–101
Wilson P, Fuller E, Forer A (1987) Irradiations of rabbit myofibrils with an ultraviolet microbeam. II. Phalloidin protects actin in solution but not in myofibrils from depolymerization by ultraviolet light. Biochem Cell Biol 65: 376–385
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bourett, T.M., Howard, R.J. Actin in penetration pegs of the fungal rice blast pathogen,Magnaporthe grisea . Protoplasma 168, 20–26 (1992). https://doi.org/10.1007/BF01332647
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01332647