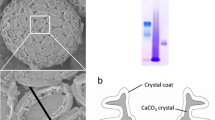
Summary
Immunolocalization of two highly acidic polysaccharides (PS-1 and PS-2) in a calcifying algaPleurochrysis carterae is described throughout the mineralization process, from before crystal nucleation through the cessation of crystal growth. This unicellular coccolithophorid alga is a useful model for mineralization because it produces calcified scales known as coccoliths in homogeneous cell culture. PS-1 and PS-2 were localized in the crystal coats of mature coccoliths and in electron dense Golgi particles. The polyanions are synthesized in medial Golgi cisternae and co-aggregate with calcium ions into discrete 25 nm particles. Particle-laden vesicles bud from cisternal margins and fuse with a coccolith-forming saccule containing an organic oval-shaped scale which forms the base of the future coccolith. The particles are localized on the base before the onset of mineral deposition and are present in the coccolith saccule throughout the period of crystal (CaCO3) nucleation and growth. During the final phase of coccolith formation, the particles disappear, and the mature crystals acquire an amorphous coat containing PS-1 and PS-2 polysaccharides which remain with the mineral phase after the coccoliths are extruded from the cell. Postulated mechanisms of polyanion-mediated mineralization are reviewed and their relevance to the calcification of coccoliths is addressed.
Similar content being viewed by others
Abbreviations
- PS-1:
-
polysaccharide one
- PS-2:
-
polysaccharide two
- BSA:
-
bovine serum albumin
- SDS:
-
sodium dodecyl sulfate
- MES:
-
2-(N-morpholino)-ethanesulfonic acid
- EDTA:
-
ethylenediaminetetraacetic acid
- DHA:
-
3-deoxy-lyxo-2-heptulosaric acid
- TCA:
-
trichloroacetic acid
References
Addadi L, Weiner S (1985) Interactions between acidic proteins and crystals: stereochemical requirements in biomineralization. Proc Natl Acad Sci USA 82: 4110–4114
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54: 484–489
Charak JHM, Pirraglia CA, Reithmeier RAF (1990) Interaction of ruthenium red with Ca2+-binding proteins. Anal Biochem 188: 123–131
Crenshaw MA (1972) The soluble matrix fromMercenaria mercenaria shell. Biomineralization 6: 6–11
— (1982) Mechanisms of normal biological mineralization of calcium carbonate. In: Nancollas GH (ed) Biological mineralization and demineralization. Springer, Berlin Heidelberg New York, pp 243–357
—, Ristedt H (1976) The histochemical localization of reactive groups in septal nacre fromNautilus pompilius L. In: Watabe N, Wilbur KM (eds) The mechanisms of mineralization in the invertebrates and plants. University of South Carolina Press, Columbia, SC, pp 355–367
Dimuzio MT, Veis A (1978 a) Phosphophoryns — major noncollagenous proteins of rat incisor dentin. Calcif Tissue Res 25: 169–178
— — (1978 b) The biosynthesis of phosphophoryns and dentin collagen in the continuously erupting rat incisor. J Biol Chem 253: 6845–6852
Domozych DS, Wells B, Shaw PJ (1991) Basket scales of the green alga,Mesostigma viride: chemistry and ultrastructure. J Cell Sci 100: 397–407
— — — (1992) Scale biogenesis in the green alga,Mesostigma viride. Protoplasma 167: 19–32
Glimcher MJ (1989) Mechanism of calcification: role of collagen fibrils and collagen-phosphoprotein complexes in vitro and in vivo. Anat Rec 224: 139–153
Green MR, Pastewka JV, Peacock AC (1973) Differential staining of phosphoproteins on polyacrylamide gels with a cationic carbocyanine dye. Anal Biochem 56: 43–51
Kyhse-Anderson J (1984) Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods 10: 203–209
Laemmli UK (1970) Cleavage of structural proteins during the assembly of bacteriophage T 4. Nature 227: 680–685
Lee SL, Veis A, Glonek T (1977) Dentin phosphoprotein: an extracellular calcium-binding protein. Biochemistry 16: 2971–2979
Linde A, Lussi A, Crenshaw MA (1989) Mineral induction by immobilized polyanionic proteins. Calcif Tissue Int 44: 286–295
Lyman J, Fleming RH (1940) Composition of sea water. J Marine Sci 3: 134–146
McKee MD, Glimcher MJ, Nanci A (1992) High-resolution immunolocalization of osteopontin and osteocalcin in bone and cartilage during endochondral ossification in the chicken tibia. Anat Rec 234: 479–492
Manton I, Leedale GF (1969) Observations on the microanatomy ofCoccolithus pelagicus andCricosphaera carterae, with special reference to their origin and nature. J Mar Biol Assoc UK 49: 1–16
—, Peterfi LS (1969) Observations on the fine structure of coccoliths, scales, and the protoplast of a freshwater coccolithophoridHymenomonas roseola Stein, with supplementary observations on the protoplast ofCricosphaera carterae. Proc R Soc Lond [Biol] 172: 1–15
Marsh ME (1986) Biomineralization in the presence of calciumbinding phosphoprotein particles. J Exp Zool 239: 207–220
— (1989) Self-association of calcium and magnesium complexes of dentin phosphophoryn. Biochemistry 28: 339–345
—, Sass RL (1983) Calcium-binding phosphoprotein particles in the extrapallial fluid and innermost shell lamella of clams. J Exp Zool 226: 193–203
— — (1984) Phosphoprotein particles: calcium and phosphate binding structures. Biochemistry 23: 1448–1456
—, Chang DK, King GC (1992) Isolation and characterization of a novel acidic polysaccharide containing tartrate and glyoxylate residues from the mineralized scales of a unicellular coccolithophorid algaPleurochrysis carterae. J Biol Chem 267: 20507–20512
Milner RE, Famulske KS, Michalak M (1992) Calcium binding proteins in the sarcoplasmic/endoplasmic reticulum of muscle and non-muscle cells. Mol Cell Biochem 112: 1–13
Outka DE, Williams DC (1971) Sequential coccolith morphogenesis inHymenomonas carterae. J Protozool 18: 285–297
Pienaar RN (1969) The fine structure ofCricosphaera carterae: I. External morphology. J Cell Sci 4: 561–567
— (1976) The rhythmic production of body covering components in the haptophycean fagellateHymenomonas carterae. In: Watabe N, Wilbur KM (eds) The mechanisms of mineralization in the invertebrates and plants. University of South Carolina Press, Columbia, SC, pp 203–229
Stetler-Stevenson WG, Veis A (1987) Bovine dentin phosphophoryn: calcium ion binding properties of a high molecular weight preparation. Calcif Tissue Int 40: 97–102
Vreeland V (1972) Immunocytochemical localization of the extracellular polysaccharide alginic acid in the brown seaweed,Fucus distichus. J Histochem Cytochem 20: 358–367
de Vrind-de Jong EW, Borman AH, Thierry R, Westbroek P, Gruter M, Kamerling JP (1986) Calcification in the coccolithophoridsEmiliania huxleyi andPleurochrysis carterae: II. Biochemical aspects. In: Leadbeater SC, Riding R (eds) Biomineralization in lower plants and animals. Clarendon Press, Oxford, pp 205–217
van der Wal P, de Jong EW, Westbroek P, de Bruijn WC, Mulder-Stapel AA (1983 a) Polysaccharide localization, coccolith formation and Golgi dynamics in the coccolithophoridHymenomonas carterae. J Ultrastruct Res 85: 139–158
— — — — (1983 b) Calcification in the coccolithophorid algaHymenomonas carterae. In: Hallberg R (ed) Environmental biogeochemistry. Swedish Research Council, Stockholm, pp 251–258 (Ecological bulletin vol 35)
— — — (1983 c) Ultrastructural polysaccharide localization in calcifying and naked cells of the coccolithophoridEmiliania huxleyi. Protoplasma 118: 157–168
Westbroek P, van der Wal P, van Emburg PR, de Vrind-de Jong EW, de Bruijn WC (1986) Calcification in the coccolithophoridsEmiliania huxleyi andPleurochrysis carterae: I. Ultrastructural aspects. In: Leadbeater SC, Riding R (eds) Biomineralization in lower plants and animals. Clarendon Press, Oxford, pp 189–203
Wheeler AP, Sikes CS (1989) Crystal matrix interactions in CaCO3 biomineralization. In: Mann S, Webb J, Williams RJP (eds) Biomineralization — chemical and biochemical perspectives. VCH, New York, pp 95–131
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marsh, M.E. Polyanion-mediated mineralization — assembly and reorganization of acidic polysaccharides in the Golgi system of a coccolithophorid alga during mineral deposition. Protoplasma 177, 108–122 (1994). https://doi.org/10.1007/BF01378985
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01378985