Abstract
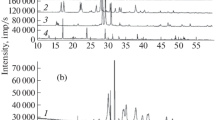
The thermal decompositions of maleates and fumarates of Cu(II) and Zn(II) have been studied by employing simultaneous non-isothermal techniques (DTG, DTA and TG). The end-products are the corresponding metal oxides, as characterized by chemical analysis and X-ray diffraction. Comparison of theTm values led to the stability sequences Cu(F)>Cu(M); Zn(F)>Zn(M) and Cu(M)≈ Cu(F); Zn(F)>Zn(M) for dehydration and decomposition, respectively.
Zusammenfassung
Die thermische Zersetzung von Maleaten und Fumaraten von Cu(II) und Zn(II) wurde mittels simultan angewandter nicht-isothermer Techniken (DTG, DTA und TG) untersucht. Die Endprodukte sind die entsprechenden Metalloxide, wie durch chemische Analyse und Röntgendiffraktometrie nachgewiesen wurde. Ein Vergleich der Tm-Werte ergab für die Stabilität in der Dehydratisierungs- bzw. Zersetzungsreaktion die Reihenfolgen Cu (F) > Cu (M); Zn (F) > Zn (M) bzw. Cu (M) ≈ Cu (F); Zn (F)>Zn (M).
Резюме
Совмещенным неизоте рмическим методом ДТ Г, ДТА и ТГ изучено термичес кое разложение малеатов и фумаратов меди и цин ка. Конечными продуктам и реакции разложения являлись окиси соотв етствующих металлов, идентифицированных химическим анализом и рентгенофазовым ана лизом. Согласно значе ниямT m , устойчивость компле ксов располагается в ряд Cu (F)>Cu (M); Zn (F)>Zn (M) и Cu (M) ≈ Cu (F); Zn (F)>Zn (M), соотв етственно, для реакций дегидратаци и и разложения.
Similar content being viewed by others
References
H. Kambe and P. D. Garn, Thermal Analysis, John Wiley and Sons, New York 1974.
W. E. Brown, D. Dollimore and A. K. Galwey, Comprehensive Chemical Kinetics, Vol. 22, Elsevier, Amsterdam 1980.
P. S. Bassi, B. S. Randhawa and H. S. Jamwal, Thermochim. Acta, 71 (1983) 15.
P. S. Bassi, B. S. Randhawa and H. S. Jamwal, Thermochim. Acta, 86 (1985) 141.
M. J. McGinn, B. R. Wheeler and A. K. Galwey, Trans. Faraday Soc, 66 (1970) 1810.
M. J. McGinn, B. R. Wheeler and A. K. Galwey, Trans. Faraday Soc., 67 (1971) 1.
A. K. Galwey, D. M. Jamieson, M. E. Brown and M. J. McGinn, Reactions Kinetics in Heterogeneous Chemical Systems, Elsevier, Amsterdam 1975, p. 520.
I. A. Vogel, Quantitative Inorganic Analysis, Longmans, Green, London 1962, pp. 531, 533.
H. E. Swanson and E. Tatge, NBS File, 4 (1949) 836.
H. E. Swanson and E. Tatge, NBS Circ. 539, Vol. 1, 1953, p. 95.
X-ray Diffraction Data for CuO, ASTM Card No. 5-0661.
H. E. Swanson and R. K. Fuyat, NBS Circ. 539, Vol. 2, 1953, p. 65.
F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, Interscience Publ., New York 1972, p. 114.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bassi, P.S., Randhawa, B.S., Khajuria, C.M. et al. Comparative study of the thermal analyses of some transition metal(II) maleates and fumarates. Journal of Thermal Analysis 32, 569–577 (1987). https://doi.org/10.1007/BF01912710
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01912710