Abstract
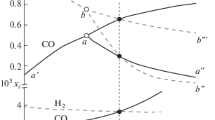
The kinetics of the chemical reaction-controlled reduction of iron oxides by H2/H2O and CO/CO2 gas mixtures are discussed. From an analysis of the systems it is concluded that the decomposition of the oxides takes place by the two dimensional nucleation and lateral growth of oxygen vacancy clusters at the gas/oxide interface. The rates of decomposition of the oxides under conditions of chemical reaction control are dependent not only on the partial pressures of the reacting gases at the reaction temperature but also on the oxygen activity of the prevailing atmosphere. Application of this model to the kinetic data leads to the determination of the maximum chemical reaction rate constants for the decomposition of the iron oxide surfaces. Assuming the reactions H2 (g) + O(ads) → H2O(g) andCO(g) + O(ads) → CO2 (g) to be rate controlling the maximum chemical reaction rate constants for the reduction of iron oxides are given by
and
The maximum chemical reaction rate constants do not necessarily indicate the maximum rates which can be achieved in practice since these will depend on the limitations imposed by mass transport in the systems. The rate constants are important however since they indicate for the first time the upper limit of any reduction rate in these systems. The fractions of reaction sites which appear to be active on wüstite surfaces in equilibrium with iron are calculated. A direct relationship between chemical reaction rates on liquid iron surfaces and rates on atomically rough iron oxide surfaces is postulated.
Similar content being viewed by others
References
C. Wagner:Advances in Catalysis, vol. 21, pp. 323–81, Academic Press, 1970.
E. T. Turkdogan and J. V. Vinters:Met. Trans., 1972, vol. 3, pp. 1561–74.
W. Pluschkell and H. Yoshikoshi:Arch. Eisenhuettenw., 1970, vol. 41, pp. 715–21.
H. Yoshikoshi, M. Tokuda, and M. Ohtani:J.J.I.M., 1972, vol. 36, pp. 1093–2000.
W. Pluschkell and B. V. S. Sarma:Arch. Eisenhuettenw., 1973, vol. 44, pp. 161–66.
Y. K. Rao:Scr. Met., 1974, vol. 8, pp. 877–82.
P. R. Swann and N. J. Tinghe:Met. Trans. B, 1977, vol. 8B, pp. 479–87.
J. R. Porter and P. R. Swann:Ironmaking and Steelmaking, 1977, vol. 4, pp. 300–07.
P. Hayes and P. Boswell: Unpublished research, Department of Mining and Metallurgical Engineering, University of Queensland, Brisbane, 1978.
P. Hayes and P. Grieveson: Final report, British Steel Corporation Research Fellowship, British Steel Corporation, London, 1975.
W. A. Edminston and R. E. Grace:Trans. TMS-AIME, 1966, vol. 236, pp. 1547–50.
A. Gala and H. J. Grabke:Arch. Eisenhuettenw., 1972, vol. 43, pp. 463–69.
H. J. Grabke:Ber. Bunsenges., 1965, vol. 69, pp. 48–57.
E. Riecke and K. Bohnenkamp:Arch. Eisenhuettenw., 1969, vol. 40, pp. 717–25.
R. A. Giddings and R. S. Gordon:J. Amer. Ceram. Soc, 1973, vol. 56, pp. 111–16.
J. P. Hirth:Energetics in Metallurgical Phenomena, William M. Mueller, ed., vol. 2, pp. 3-52, Gordon and Breach, N.Y.
K. A. Jackson:Liquid Metals and Solidification, p. 156, ASM, Metals Park, Ohio, 1958.
D. R. Sain and G. R. Belton:Met. Trans. B, 1976, vol. 7B, pp. 235–44.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hayes, P.C. The kinetics of formation of h2o and co2 during iron oxide reduction. Metall Trans B 10, 211–217 (1979). https://doi.org/10.1007/BF02652465
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02652465