Abstract
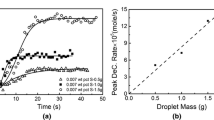
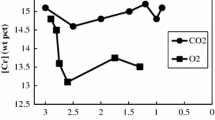
The observed retarding effect of sulfur on the decarburization of Fe-C melts has been interpreted by means of a mixed-control mechanism involving gas-phase mass transfer and dissociative adsorption of CO2. A mathematical model formulated on the basis of the proposed mechanism gave an excellent fit to the experimental data. The application of the model to the data provided a value of 4.42 x 10−3 mole · cm−2 · s−1 · atm−1 for the dissociative adsorption rate constant for CO2 on liquid iron at 1973 K; the fraction of surface sites that cannot be occupied by sulfur, even at apparent surface-saturation, was found to be 0.085. The model predicts a residual rate of decarburization at high sulfur concentrations; this prediction is borne out by the experiment. The effect of convective motion within the levitated melt on the rate of decarburization below a characteristic carbon concentration was quantified. The liquid-phase mass transfer was greatly enhanced by the stirring effect of the electromagnetic field. The effective diffusivity of carbon in Fe-C melts under levitation conditions has been found to be 3.24 x 10−3 cm2 · s−1, a value ten times as large as that under stationary conditions.
Similar content being viewed by others
References
L.A. Baker, N.A. Warner, and A.E. Jenkins:Trans. TMS-AIME, 1964, vol. 230, pp. 1228–35.
L.A. Baker, N.A. Warner, and A.E. Jenkins:Trans. TMS-AIME, 1967, vol. 239, pp. 857–66.
P. A. Distin, G. D. Hallett, and F. D. Richardson:J. Iron Steel Inst., London, 1968, vol. 206, pp. 821–33.
F. D. Richardson: inChemical Metallurgy of Iron and Steel, The Iron and Steel Institute, London, 1973, pp. 82–92.
R.L. Steinberger and R.E. Treybal:A.I.Ch.E.J., 1960, vol. 6, no. 2, pp. 227–32.
F. D. Richardson:Physical Chemistry of the Melts in Metallurgy, Academic Press, New York, NY, 1974, vol. 2, p. 463.
J. Crank:The Mathematics of Diffusion, Oxford University Press, London, 1956, pp. 84–98.
H.J. Grabke:Ber Bunseng, 1966, vol. 70, p. 664.
K. Suzuki and K. Mori:Trans. Iron Steel Inst. Jpn., 1977, vol. 17, pp. 136–42.
D.R. Sain and G. R. Belton:Metall. Trans. B, 1978, vol. 9B, pp. 235–44.
J. T. Davies and C. R. A. Mayers:Chem. Engng. Sci., 1961, vol. 16, pp. 55–68.
J.T. Davies and W. Khan:Chem. Engng. Sci., 1965, vol. 20, pp. 713–15.
H.J. Grabke:Proc. Third Intl. Congress on Catal., North Holland, Amsterdam, 1965, pp. 928–98.
R.J. Freuhan and L.J. Martonik:High Temp. Sci., 1971, vol. 3, pp. 244–56.
H. Nomura and K. Mori:Trans. Iron Steel Inst. Jpn., 1973, vol. 13, pp. 265–73.
M. Hayer and S.G. Whiteway:Can. Metall. Quart., 1973, vol. 12, no. 1, pp. 35–44.
J.P. Morris and R.C. Buehl:Trans. AIME, 1950, vol. 188, pp. 317–22.
P. Kozakevitch, S. Chatel, G. Urbain, and M. Sage:Rev. Met., 1955, vol. 52, p. 139.
Y. Wanibe, S. Takai, T. Kojima, and H. Sakao:Trans. Iron Steel Inst. Jpn., 1980, vol. 20, no. 11, pp. 783–89.
E. W. Washburn:International Critical Tables of Numerical Data, Physics, Chemistry and Technology, McGraw-Hill, New York, NY, 1928, vol. III, p. 208.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, H.G., Rao, Y.K. Rate of decarburization of iron-carbon melts: Part II. a mixed-control model. Metall Trans B 13, 411–421 (1982). https://doi.org/10.1007/BF02667757
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02667757