Abstract
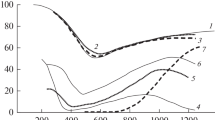
The chemical reactions of dense pellets of solid CaS in oxidizing atmospheres were studied by using continuous thermogravimetric analysis and iodimetric titration of SO2 in the off-gas. The experiments covered a temperature range of 1223 to 1853 K, and Ar-O2 mixtures varying from 1 to 100 pct O2. The oxidation of CaS was found to be a complex process involving the formation of CaO and CaSO4. Within the experimental conditions stated, three different processes were observed: 1) Weight loss with the formation of CaO and SO2 which occurred at high temperatures and low partial pressures of O2. 2) Weight gain which occurred at low temperatures and high partial pressures of O2. 3) Combination of a) weight gain with the overall formation of CaSO4 and b) weight loss with the overall oxidation of CaS to CaO and the decomposition of CaSO4 leading to an oscillatory behavior which occurred at intermediate temperatures and intermediate to high partial pressures of O2. The boundaries between these three processes are distinct. The rate of oxidation of the sulfide is limited first by the transport of O2 across the gas boundary layer and later by the diffusion of O2 through the porous reaction product. From these results and the Ca-S-0 stability diagram, it is possible to predict the experimental conditions which will produce processes of weight gain, weight loss and oscillatory behavior.
Similar content being viewed by others
References
J. Zawadski, I. Kowalczenski, and St. Zeromski:Rocz. Chem., 1928, vol. 8, p. 358.
R. Curtis:Gazz. Chim. liai., 1938, vol. 68, p. 699.
K. Schitzgebel and P. S. Lowell:Environ. Sci. Technol., 1973, vol. 7, pp. 1147–51.
E. S. Newman:J. Res. Nat. Bur. Stand., 1941, vol. 27, pp. 191–96.
E. W. Dewing and F. D. Richardson:Trans. Faraday Soc, 1959, vol. 55, pp. 611–15.
W. Gutt and M. A. Smith:Trans. Br. Ceram. Soc, 1967, vol. 66, pp. 337–45.
P. Grievson and E. T. Turkdogan:Trans. TMS-AIME, 1962, vol. 224, pp. 1086–95.
E. T. Turkdogan, B. B. Rice and J. V. Vintners:E. T. Turkdogan, B. B. Rice and J. V. VintnersMet. Trans., 1974, vol. 5, pp. 1527–35.
G. J. W. Kor and F. D. Richardson:Trans. Inst. Min. Metall, Sect. C, 1970, vol. 79, pp. C148–56.
T. Rosenqvist:J. Met., 1951, vol. 4, pp. 535–40.
D. C. Lynch and J. F. Elliott:Met. Trans. B, 1978, vol. 9B, pp. 691–704.
J. Szekely, M. Choudhary, and Y. El-Tawil:Met. Trans. B., 1977, vol. 8B, pp. 639–43.
H. W. Hsu and R. B. Bird:AIChEJ., 1960, vol. 6, pp. 516–24.
D. R. Stull,et. al ANAF Thermochemical Tables, loose-leaf edition, Dow Chemical Co., Midland, MI 1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lynch, D.C., Elliott, J.F. Analysis of the oxidation reactions of CaS. Metall Trans B 11, 415–425 (1980). https://doi.org/10.1007/BF02676885
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02676885