Abstract
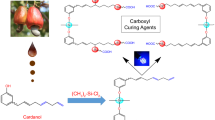
We have investigated the reactions of glycidyl ether, glycidyl ester, and other oxirane functional resins with carboxyl or anhydride functional compounds and polymers in the presence of a wide range of amine, phosphonium, and metal catalysts.
We confirmed that both amine and phosphonium compounds can catalyze the reaction of epoxy groups with carboxyl and anhydride groups. There are certain deficiencies with these catalysts, such as a tendency to yellow and a reduction in stability at ambient or elevated temperatures. We also observed that many of the known amine catalysts contribute to poorer humidity resistance and exterior durability. Several metal salts were found to be effective catalysts, but they also contributed to a reduction in chemical resistance or they led to paint instability.
We have discovered a group of metal chelates that overcome these problems and provide stable formulations in a single package that do not yellow during cure and that give improved resistance properties. The new catalysts have been evaluated in high-solids epoxy/carboxyl coatings, automotive clearcoats, and powder coatings.
Similar content being viewed by others
References
May, C.A.,Epoxy Resins Chemistry and Technology, Marcel Dekker (1988).
Cycloaliphatic Epoxy Dow Chemical ERL-4221 or Daicel Chemical Celoxide 2021.
Hildon, A.M. and Greenhalgh, P.F., Interox Chemicals, U.S. Patent 4,071,541 (1976).
McLafferty, J.J. and Wang, S.L., Ruco Polymer Corporation, U.S. Patent 4,910,287 (1990).
Victorius, C., E.I. Du Pont de Nemours and Co., U.S. Patent 4,027,066 (1977).
Shecter, L., Wynbtra, J., and Kurkjy, R.E.,Ind. Eng. Chem., 49, 1107 (1957).
Murai, K., Akazone, G., and Murakami, Y.,Kogyo Kagaku Zasshi, 63, 283 (1960).
Matejka, L. and Dusek, K.,Polym. Bull. (Berlin), 15(3), 215–21 (1986).
Kyuma, T., Akazome, G., Murai, M., Sakai, S., and Ishii, Y.,Kogyo Kagaku Zasshi, 70, 169 (1970).
Briggs, R.L., Campbell, D.H., and Montagne, M.R., (BASF Corp.). Eur. Pat. Appl. EP 602559 (1994).
McEntire, E.F., Claar, J.A., Thomas, S.J., Walters, D.N., David, N. (PPG Industries, Inc.). PCT Int. Appl. WO 9214786 A1 3 Sep (1992).
Van de Ven, G.J., Leijzer, R.T.M., Brinkman, E., and Vandevoorde,Double Liaison—Phys., Chim. Econ. Peint. Adhes., 44(498–499), 67–71 (1997).
Carey, J.E. and Reilly, L.C., Shell Oil Co., U.S. Patent 4,069,203 (1978).
Marten, M., Fink, D., and Godau, C., (Hoechst A.-G., Germany). Eur. Pat. Appl. EP 617069 (1994).
Lee, H. and Neville, K., “Mater. Symp., Nat’l. Soc. Aerospace Mater. Process Engrs. 7th Symp.” Los Angeles, 1964: 2/14.
Fish, W. and Hofmann, W.,J. Polym. Sci., 12:497 (1954).
Fish, W., Hofmann, W., and Koskikallio,Chem. Ind. (London) 1956, 756.
Nakane, Y., Mizutani, H., Ishibashi, H., and Ishidoya, M., Latent Acid Catalyst U.S. Patent 5,661,219 (1997).
Connelly, W. and McEwan, I.H., Canadian Industries, ZA 6907152 (1971).
Nakane, Y., Ishidoya, M., and Endo, T., Nettowaku Porima, 19(4), 228–235 (1998).
Mizutani, H., Nakane, Y., and Ishidoya, M., PCT Int. Appl, WO 9639454.
Kastens, A.S., inPolyethers, Part 1, Interscience, New York, 1963.
Maeda, Katsuyuki, Ikui, Sozo, and Harano, Yoshuki (Daicel Chem, Japan). Jpn. Kokai Tokkyo Koho JP 06207126 (1994).
Wright, A.J., O’Hara, K.J., and Turner, S.K., Coats Brothers, U.S. Patent 4558076 (1985).
Ishii Y. and Sakai, S., inRing Opening Polymerization, Dekker, NY, p. 12, 1969.
Furukawa, J. and Saegusa, T., “Polymerization of Aldehydes and Oxides,” Interscience, NY, 1963.
NACURE® XC-9206 zinc chelate catalyst from King Industries, Norwalk, CT, Zn content 10 %, supplied in aliphatic hydrocarbon solvent.
GMA 207-SA glycidyl functional acrylic polymer from Reichhold with an epoxy equivalent weight of 490 based on resin solids.
Joncryl 819 acrylic polymer (Johnson Polymer) with a carboxyl equivalent weight of 748.
b* value (yellow index) measured with Minolta Spectrophotometer 508d according to CIELAB. CIE stands for Comission Internationale de l’Eclairage (International Commission on Illumination.) More information available from Color Vision Laboratory San Diego, www-cvrl.ucsd.edu.
Mono and Di alkyl ester of a poly acid with pka values of between 2 and 13, respectively. He, Z.A., Blank, W.J., and Picci, M., U.S. Patent 6,335,304B1 (2002).
Fine Clad M-8841 is a carboxyl functional polyester from Reichhold Chemical with an carboxyl equivalent weight of 1020.
Epon 828 is a product of Resolution Performance Products, bisphenol A diglycidylether with an epoxy equivalent weight of 188.5.
Epon 1001, available from Resolution Performance Products Co. (100 % solids, epoxy equivalent weight=538).
Ti-Pure R-900 a titanium dioxide pigment from E.I. DuPont de Nemours, Wilmington, DE.
Vitroflex 7170 available from of Elf Atochem, (oxirane equivalent weight 228, oxirane content 7.0%).
Author information
Authors and Affiliations
Additional information
Science Rd, Norwalk, CT 06852.
Rights and permissions
About this article
Cite this article
Blank, W.J., He, Z.A. & Picci, M. Catalysis of the epoxy-carboxyl reaction. Journal of Coatings Technology 74, 33–41 (2002). https://doi.org/10.1007/BF02720158
Issue Date:
DOI: https://doi.org/10.1007/BF02720158