Abstract
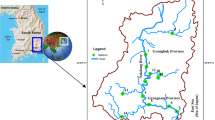
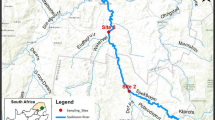
A study was conducted to investigate the trace metal pollution of water and sediments of downstream of Tsurumi River, Yokohama, Japan. Twenty samples of water and sediments were collected from the river starting from Tokyo bay side up to the junction point of the Yagami River. Results show that the mean concentrations of chromium, cupper and nickel in water greatly exceed (>100 times) the surface water standard. The concentration of molybdenum and lead was also higher than standard values while iron and manganese was lower than that of surface water standard. The mean concentration of zinc, cupper, cadmium, lead, chromium, vanadium, bromine and iodine was 381.1, 133.0, 1.0, 40.8, 102.9, 162.0, 71.5 and 10.6 μg/g sediments, respectively and was greatly exceed the average worldwide shale concentrations and average Japanese river sediment values. However, mean concentration of arsenic, nickel and strontium was 11.0, 36.6 and 164.6 μg/g sediments, respectively which was lower than the average shale value. Other analyzed trace metals, including barium, zirconium, rubidium, yttrium, tin, antimony, cesium, lanthanum, cerium, praseodymium and neodymium were detected in river sediments; the concentration of which was close to the Japan’s river sediment average values. Pollution load index values of the sites of the studied area ranged from 1.24 to 7.65 which testify that the river sediments are polluted. The PLI value of the area was, however, high (6.53) as the concentration of trace metals like zinc, cupper, cadmium, lead and chromium were very high and were the major pollutants.
Similar content being viewed by others
References
Abdel-Ghani, N. T.; Elchaghaby, G. A., (2007). Influence of operating conditions on the removal of Cu, Zn, Cd and Pb ions from wastewater by adsorption. Int. J. Environ. Sci. Tech., 4 (4), 451–456 (6 pages).
Abdel-Ghani, N. T.; Hefny, M.; El-Chaghaby, G. A. E, (2007). Removal of lead from aqueous solution using low cost abundantly available adsorbents. Int. J. Environ. Sci. Tech., 4 (1), 67–74 (8 pages).
Agbozu, I. E.; Ekweozor, I. K. E.; Opuene, K., (2007). Survey of heavy metals in the catfish synodontis clarias. Int. J. Environ. Sci. Tech., 4 (1), 93–98 (6 pages).
Akcay, H.; Oguz, A; Karapire, C., (2003). Study of heavy metal pollution and speciation in Buyak Menderes and Gediz river sediments. Water Res., 37 (4), 813–822 (10 pages).
Apha, (1998). Standard methods for the examination of water and wastewater. 20th. Ed., published jointly by American Public Health Association, American Water Works Association, Water Pollution Control Federation, Washington D.C.
Atgin, R. S.; El-Agha, O.; Zararsiz, A.; Kocatas, A.; Parlak, H.; Tuncel, G., (2000). Investigation of the sediment pollution in Izmir Bay: trace elements. Spectrochim. Acta B., 55 (7), 1151–1164 (14 pages).
Cenci, R. M.; Martin, J. M., (2004). Concentration and fate of trace metals in Mekong River Delta. Sci. Total Environ., 332 (1–3), 167–182 (16 pages).
Das, J. D.; Nolting, R. E, (1993). Distribution of trace metals in sediments and pore waters in the N.W. Mediterranean Sea. NIOZ, EROS-200 Project, 10.
Dassenakis, M.; Scoullos, M.; Foufa, E.; Krasakopoulou, E.; Pavlidou, A.; Kloukiniotou, M., (1998). Effects of multiple source pollution on a small Mediterranean River. Appl. Geochem., 13 (2), 197–211 (15 pages).
Environment Canada, (2002). Canadian sediment quality guidelines for the protection of aquatic life: Summary table. http://www.doeal.gov/SWEIS/OtherDocuments/328%20envi%20canada%202002.pdf
Forstner, U., (1981). Metal pollution assessment from sediment analysis. in: Forstner, U. and Wittmann, G. T. W. (Eds.) Metal pollution in the aquatic environment. Springer, Berlin Heidelberg, New York, 486.
Gamo, T., (2007). Environmental geochemistry. (In Japanese) Baihu-kan, Japan, 118–119.
Harikumar, P. S.; Nasir, U. P.; Mujeebu Rahman, M. P., (2009). Distribution of heavy metals in the core sediments of a tropical wetland system. Int. J. Environ. Sci. Tech., 6 (2), 225–232 (8 pages).
Hobbelen, P. H. F.; Koolhaas, J. E.; van Gestel, C. A. M., (2004). Risk assessment of heavy metal pollution for detritivores in floodplain soils in the Biesbosch, the Netherlands, taking bioavailability into account. Environ. Pollut, 129 (3), 409–419 (11 pages).
Horowitz, A. J., (1991). A primer on sediment-trace element chemistry. 2nd. Ed., Lewis Publishers, Chelsea (Michigan), 136.
Huheey, J. E., (1983). Inorganic chemistry: Principles of structure and reactivity. Harper and Row Publishers, New York, 912.
Islam, M. R.; Lahermo, W. P.; Salminen, R.; Rojstaczer, S.; Peuraniemi, V., (2000). Lake and reservoir water quality affected by metals leaching from tropical soils, Bangladesh. Environ. Geol., 39 (10), 1083–1089 (8 pages).
Jaquet, J. M.; Davaud, E.; Rapin, F.; Vernet, J. P., (1982). Basic concepts and associated statistical methodology in geochemical study of lake sediments. Hydrobiologia, 91 (1), 139–146 (8 pages).
Jones, D. S.; Sutter, I. L; G W.; Hull, R. N., (1997). Toxicological benchmarks for screening contaminants of potential concern for effects on sediment-associated biota: 1997 Revision, ES/ ER/TM-95/R4. Oak Ridge National Laboratory, prepared for the US Department of Energy. http://www.ornl.gov/~webworks/cpr/rpt/68667.pdf
Karbassi, A. R.; Monavari, S. M.; Bidhendi, G. R. N.; Nouri, J.; Nematpour, K., (2008). Metal pollution assessment of sediment and water in the Shur River. Environ. Monit. Assess., 147 (1–3), 107–116 (10 pages).
Khan, A. H.; Nolting, R. F.; Vander Gaast, S. J.; Van Raaphorst, W., (1992). Trace element geochemistry at the sediment water interface in the North Sea and the Western Wadden Sea. Netherlands Institute for Sea Research. NIOZ Report 1992–10. BARC Report 1992-1, BEON Report 18.
Kwon, Y. T.; Lee, C. W.; Ahn, B. Y, (2001). Sedimentation pattern and sediments bioavailability in a wastewater discharging area by sequential metal analysis. Microchem. J.,68 (2–3), 135–141 (7 pages).
Legret, M.; Pagotto, C., (1999). Evaluation of pollutant loadings in the runoff waters from a major rural highway. Sci. Total Environ., 235, 143–150 (8 pages).
Marin, A.; Lopez-Gonzalvez, A.; Barbas, C., (2001). Development and validation of extraction methods for determination of zinc and arsenic speciation in soils using focused ultrasound: Application to heavy metal study in mud and soils. Anal. Chim. Acta., 442 (2), 305–318 (14 pages).
Miller, C. V.; Foster, G. D.; Majedi, B. F., (2003). Baseflow and stormflow metal fluxes from two small agricultural catchments in the coastal plain of Chesapeake Bay Basin, United States. Appl. Geochem., 18 (4), 483–501 (19 pages).
Millero, F. J., (1974). The physical chemistry of seawater. Ann. Rev. Earth Planet. Sci., 2, 101–150 (50 pages).
MOE, (2004a). Ministry of Environment, Japan. Environmental quality standards for water pollution. Godochosha No. 5, 1-2-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-8975, Japan. http://www.env.go.jp/en/water/wq/wp.pdf
MOE, (2004b). Ministry of Environment, Japan. Environmental quality standards for soil pollution. Godochosha No. 5, 1-2-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-8975, Japan. http:// www.env.go.jp/en/water/soil/sp.html
Mukai, H.; Tanaka, A.; Fujii, T.; Nakao, M., (1994). Lead isotope ratios of airborne particulate matter as tracers of long-range transport of air pollutants around Japan. J. Geophys. Res., 99 (2), 3717–3726 (10 pages).
Muller, G., (1969). Index of geoaccumulation in sediments of the Rhine River. Geo. J., 2 (3), 108–118 (11 pages).
Muller, G, (1981). Die Schwermetallbelstung der Sedimente des Neckars und seiner Nebenflusse: eine estandsaufnahme. Chem. Zeitung, 105, 157–164 (8 pages).
Nicolau, R.; Galera-Cunha, A.; Lucas, Y., (2006). Transfer of nutrients and labile metals from the continent to the sea by a small Mediterranean river. Chemosphere, 63 (3), 469–476 (8 pages).
Nito, S.; Kanno, Y.; Muto, A.; Uesugi, A.; Nakahama, T.; Inouye, Y., (2003). Biological evaluation of the pollution of the Tsurumi River with 7-Ethoxycoumario O-Deethylase activity induced by river sediment extracts in HepG2 cells. J. Health Sci., 49 (1), 8–12 (5 pages).
OMOE, (1993). Ontario Ministry of Environment, Guidelines for the protection and management of aquatic sediment quality in Ontario. Ontario Ministry of the Environment, Canada, 3 http://www.ene.gov.on.ca/envision/gp/B1-3.pdf
Omori, M.; Hatayama, Y.; Horiguchi, M., (Eds.), (1986). Geology of Japan, Kanto Districts (in Japanese). 1st. Ed. Kyoritsu Publishing Co., Tokyo, Japan, 350.
Osmond, D. L.; Line, D. E.; Gale, J. A; Gannon, R. W.; Knott, C. B.; Bartenhagen, K. A.; Turner, M. H.; Coffey, S. W.; Spooner, J.; Wells, J.; Walker, J. C.; Hargrove, L. L.; Foster, M. A.; Robillard, P. D.; Lehning, D. W., (1995). Water, Soil and Hydro-Environmental Decision Support System. http://www.water.ncsu.edu/watersheds/info/hmetals.html
Sheikh, M. A.; Noah, N. M.; Tsuha, K.; Oomori, T., (2007). Occurrence of tributyltin compounds and characteristics of heavy metals. Int. J. Environ. Sci. Tech., 4 (1), 49–60 (12 pages).
Shrestha, R.; Fischer, R.; Sillanpaa, M., (2007). Investigations on different positions of electrodes and their effects on the distribution of Cr at the water sediment interface. Int. J. Environ. Sci. Tech., 4 (4), 413–420 (7 pages).
Sorme, L.; Lagerkvist, R., (2002). Sources of trace metals in urban wastewater in Stockholm. Sci. Total Environ., 298 (1–3), 131–145 (15 pages).
Stoeppler, M., (1991). Cadmium. in: Merian E. (Ed.), Metals and their compounds in the environment: Occurrence, analyses and biological relevance. VCH, New York. 803–851 (49 pages).
Taylor, S. R., (1964). Abundances of chemical elements in the continental crust: A new table. Geochim. Cosmochim. Acta.,28 (8), 1273–1285 (13 pages).
Tessier, A.; Campbell, P. G C; Bisson, M., (1979). Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem., 51 (7), 844–851 (8 pages).
Tomlinson, D. C.; Wilson, J. G; Harris, C. R.; Jeffrey, D. W., (1980). Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoland Mar. Res., 33, 566–575 (8 pages).
Turekian, K. K.; Wedepohl, K. H. (1961). Distribution of the elements in some major units of the earth’s crust. Geol. Soc. Am. Bull., 72, 175–192 (16 pages).
US EPA., (1989). U.S. Environmental Protection Agency. National Interim Primary Drinking Water Regulations. Code of Federal Regulations, Title 40, Parts 141 and 142.
US EPA., (1999). U.S. Environmental Protection Agency. Screening level ecological risk assessment protocol for hazardous waste combustion facilities. Vol. 3, Appendix E: Toxicity reference values. EPA530-D99-001C. http://www.epa.gov/epaoswer/hazwaste/combust/eco-risk/voume3/appx-e.pdf
Walkley, A.; Black, I. A., (1934). An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci., 37, 29–38 (10 pages).
Wedepohl, K. H., (1969–1979). Handbook of geochemistry. Vols. I and II, Springer, Berlin Heidelberg, New York.
Zakir, H. M.; Sharmin, S.; Shikazono, N., (2006). Heavy metal pollution in water and sediments of Turag river at Tongi area of Bangladesh. Int. J. Lake River., 1 (1), 85–96 (12 pages).
Zvinowanda, C. M.; Okonkwo, J. O.; Shabalala, P. N.; Agyei, N. M., (2009). A novel adsorbent for heavy metal remediation in aqueous environments. Int. J. Environ. Sci. Tech., 6 (3), 425–434 (11 pages).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohiuddin, K.M., Zakir, H.M., Otomo, K. et al. Geochemical distribution of trace metal pollutants in water and sediments of downstream of an urban river. Int. J. Environ. Sci. Technol. 7, 17–28 (2010). https://doi.org/10.1007/BF03326113
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03326113