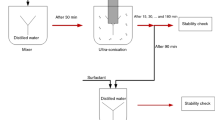
Abstract
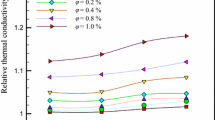
An extensive experimental investigation on the thermophysical properties of two different types of R718 (water) based nanofluid was performed for particle volume fractions (ϕ) ranging from 0.25–2.5%. R718 (water) is used as a secondary refrigerant in refrigeration systems; therefore, it is important to measure the variation in the thermophysical properties of R718 due to the addition of nanoparticles. The major highlight of the current work is the measurement of the thermophysical properties at a temperature as low as 278 K. Most of the studies available in the open literature are based on the thermophysical property measurement of nanofluids at elevated temperatures (above 293 K). In the present work, the thermophysical properties such as thermal conductivity, viscosity and density of the nanofluids were measured at a temperature varying from 5 to 25 °C (278-298 K) and were later compared with the existing theoretical models and previous experimental results. The specific heat capacity of Al2O3 and CuO based nanofluids was predicted and analysed using a theoretical model. The thermal conductivity of the nanofluid was measured by the Transient hot-wire method and the viscosity of the nanofluid was measured using a Cannon-Fenske viscometer. Copper oxide (CuO) and aluminium oxide (Al2O3) nanoparticles were chosen for the present investigation. The average particle diameter of CuO and Al2O3 nanoparticles was 30 nm. It was observed that the enhancement in thermal conductivity of nanofluids at low temperature (~278 K) is considerably lower than the enhancements at elevated temperatures. The viscosity measurements also revealed that the classical models can be successfully applied for predicting the viscosity of Al2O3 –R718 nanofluids at low particle volume fractions (ϕ < 0.5%).









Similar content being viewed by others
Abbreviations
- C :
-
Euler’s constant
- C 1 :
-
Empirical constant
- C 2 :
-
Empirical constant
- C p :
-
Specific heat capacity [J/kg.K]
- d:
-
Diameter [m]
- I :
-
Current [A]
- k :
-
Thermal conductivity [W/m.K]
- k B :
-
Boltzmann constant [m2kg/s K]
- m :
-
Mass [kg]
- q :
-
Heat dissipated per unit length [W/m]
- r :
-
Radius [m]
- R :
-
Resistance [ohms]
- T :
-
Temperature [K]
- t :
-
Time (s)
- V :
-
Volume [m3]
- V B :
-
Brownian velocity [m/s]
- V un :
-
Unbalanced wheatstone bridge voltage [V]
- ΔT :
-
Change in wire temperature [K]
- μ :
-
Dynamic viscosity [cP]
- α :
-
Thermal diffusivity [m2 /s]
- ν :
-
Kinematic viscosity [cSt]
- ρ :
-
Density [kg/m3]
- ϕ :
-
Particle volume fraction [%]
- φ :
-
Particle volume fraction
- ψ :
-
Particle shape factor
- Br:
-
Brownian
- cl:
-
cluster
- f:
-
Base fluid
- i:
-
Initial
- nf:
-
Nanofluid
- np:
-
Nanoparticle
- un:
-
Unbalanced
- w:
-
Wire
- ANN:
-
Artificial neural network
- EG:
-
Ethylene glycol
- THW:
-
Transient hot wire
References
Cheng L, Liu L (2013) Boiling and two-phase flow phenomena of refrigerant-based nanofluids: Fundamentals, applications and challenges. Int J Refrig 36:421–446. https://doi.org/10.1016/j.ijrefrig.2012.11.010
Nair V, Tailor PR, Parekh AD (2016) Nanorefrigerants: A comprehensive review on its past, present and future. Int J Refrig 67:290–307. https://doi.org/10.1016/j.ijrefrig.2016.01.011
Peng H, Ding G, Hu H, Jiang W (2011) Effect of nanoparticle size on nucleate pool boiling heat transfer of refrigerant/oil mixture with nanoparticles. Int J Heat Mass Transf 54:1839–1850. https://doi.org/10.1016/j.ijheatmasstransfer.2010.12.035
Sözen A, Özbaş E, Menlik T (2014) Improving the thermal performance of diffusion absorption refrigeration system with alumina nanofluids: An experimental study. Int J Refrig 44:73–80. https://doi.org/10.1016/j.ijrefrig.2014.04.018
Mahbubul IM, Fadhilah SA, Saidur R et al (2013) Thermophysical properties and heat transfer performance of Al 2O3/R-134a nanorefrigerants. Int J Heat Mass Transf 57:100–108. https://doi.org/10.1016/j.ijheatmasstransfer.2012.10.007
Li CH, Peterson GP (2007) The effect of particle size on the effective thermal conductivity of Al2O3-water nanofluids. J Appl Phys 101:044312. https://doi.org/10.1063/1.2436472
Karthikeyan NR, Philip J, Raj B (2008) Effect of clustering on the thermal conductivity of nanofluids. Mater Chem Phys 109:50–55. https://doi.org/10.1016/j.matchemphys.2007.10.029
Ghanbarpour M, Bitaraf Haghigi E, Khodabandeh R (2014) Thermal properties and rheological behavior of water based Al2O3 nanofluid as a heat transfer fluid. Exp Thermal Fluid Sci 53:227–235. https://doi.org/10.1016/j.expthermflusci.2013.12.013
Patel HE, Sundararajan T, Das SK (2010) An experimental investigation into the thermal conductivity enhancement in oxide and metallic nanofluids. J Nanopart Res 12:1015–1031. https://doi.org/10.1007/s11051-009-9658-2
Lee JH, Hwang KS, Jang SP, Lee BH, Kim JH, Choi SUS, Choi CJ (2008) Effective viscosities and thermal conductivities of aqueous nanofluids containing low volume concentrations of Al2O3 nanoparticles. Int J Heat Mass Transf 51:2651–2656. https://doi.org/10.1016/j.ijheatmasstransfer.2007.10.026
Kolsi L (2016) Numerical analysis of periodic 3D convective heat transfer in fenestration with between-The-glass louvered blinds. Case Stud Therm Eng 8:71–83. https://doi.org/10.1016/j.csite.2016.05.002
Kolsi L, Mahian O, Öztop HF, Aich W, Borjini MN, Abu-Hamdeh N, Aissia HB (2016) 3D buoyancy-induced flow and entropy generation of nanofluid-filled open cavities having adiabatic diamond shaped obstacles. Entropy 18:232. https://doi.org/10.3390/e18060232
Das SK, Choi SUS, Yu W, Pradeep T (2007) Nanofluids: Science and Technology, 1st edn. John Wiley & Sons, New York. https://doi.org/10.1002/9780470180693
Bianco V, Manca O, Nardini S, Vafai K (2010) Heat transfer enhancement with nanofluids. CRC Press, Taylor & Francis, Boca Raton
Mintsa HA, Roy G, Nguyen CT, Doucet D (2009) New temperature dependent thermal conductivity data for water-based nanofluids. Int J Therm Sci 48:363–371. https://doi.org/10.1016/j.ijthermalsci.2008.03.009
Khedkar RS, Sonawane SS, Wasewar KL (2012) Influence of CuO nanoparticles in enhancing the thermal conductivity of water and monoethylene glycol based nanofluids. Int Commun Heat Mass Transf 39:665–669. https://doi.org/10.1016/j.icheatmasstransfer.2012.03.012
Buonomo B, Manca O, Marinelli L, Nardini S (2015) Effect of temperature and sonication time on nanofluid thermal conductivity measurements by nano-flash method. Appl Therm Eng 91:181–190. https://doi.org/10.1016/j.applthermaleng.2015.07.077
Agarwal R, Verma K, Agrawal NK, Duchaniya RK, Singh R (2016) Synthesis, characterization, thermal conductivity and sensitivity of CuO nanofluids. Appl Therm Eng 102:1024–1036. https://doi.org/10.1016/j.applthermaleng.2016.04.051
Hemmat Esfe M, Afrand M, Yan WM, Akbari M (2015) Applicability of artificial neural network and nonlinear regression to predict thermal conductivity modeling of Al2O3-water nanofluids using experimental data. Int Commun Heat Mass Transf 66:246–249. https://doi.org/10.1016/j.icheatmasstransfer.2015.06.002
Pryazhnikov MI, Minakov AV, Rudyak VY, Guzei DV (2017) Thermal conductivity measurements of nanofluids. Int J Heat Mass Transf 104:1275–1282. https://doi.org/10.1016/j.ijheatmasstransfer.2016.09.080
Nguyen CT, Desgranges F, Roy G, Galanis N, Mare T, Boucher S, Angue-Mintsa H (2007) Temperature and particle-size dependent viscosity data for water-based nanofluids - Hysteresis phenomenon. Int J Heat Fluid Flow 28:1492–1506. https://doi.org/10.1016/j.ijheatfluidflow.2007.02.004
Hamed Mosavian MT, Zeinali Heris S, Etemad SG, Nasr Esfahany M (2010) Heat transfer enhancement by application of nano-powder. J Nanopart Res 12:2611–2619. https://doi.org/10.1007/s11051-009-9840-6
Venerus DC, Buongiorno J, Christianson R et al (2010) Viscosity measurements on colloidal dispersions (nanofluids) for heat transfer applications. Appl Rheol 20:44582. https://doi.org/10.3933/ApplRheol-20-44582
Minakov AV, Rudyak VY, Pryazhnikov MI (2018) Rheological behavior of water and ethylene glycol based nanofluids containing oxide nanoparticles. Colloids Surf A Physicochem Eng Asp 554:279–285. https://doi.org/10.1016/j.colsurfa.2018.06.051
Pastoriza-Gallego MJ, Casanova C, Legido JL, Piñeiro MM (2011) CuO in water nanofluid: Influence of particle size and polydispersity on volumetric behaviour and viscosity. Fluid Phase Equilib 300:188–196. https://doi.org/10.1016/j.fluid.2010.10.015
Meybodi MK, Daryasafar A, Koochi MM et al (2016) A novel correlation approach for viscosity prediction of water based nanofluids of Al2O3, TiO2, SiO2 and CuO. J Taiwan Inst Chem Eng 58:19–27. https://doi.org/10.1016/j.jtice.2015.05.032
Vajjha RS, Das DK, Mahagaonkar BM (2009) Density measurement of different nanofluids and their comparison with theory. Pet Sci Technol 27:612–624. https://doi.org/10.1080/10916460701857714
Nabati Shoghl S, Jamali J, Keshavarz Moraveji M (2016) Electrical conductivity, viscosity, and density of different nanofluids: An experimental study. Exp Thermal Fluid Sci 74:339–346. https://doi.org/10.1016/j.expthermflusci.2016.01.004
Pastoriza-Gallego MJ, Casanova C, Páramo R et al (2009) A study on stability and thermophysical properties (density and viscosity) of Al2O3 in water nanofluid. J Appl Phys 106:064301. https://doi.org/10.1063/1.3187732
Cabaleiro D, Gracia-Fernández C, Legido JL, Lugo L (2015) Specific heat of metal oxide nanofluids at high concentrations for heat transfer. Int J Heat Mass Transf 88:872–879. https://doi.org/10.1016/j.ijheatmasstransfer.2015.04.107
Zhou SQ, Ni R (2008) Measurement of the specific heat capacity of water-based Al2 O3 nanofluid. Appl Phys Lett 92:093123. https://doi.org/10.1063/1.2890431
Sundar LS, Farooky MH, Sarada SN, Singh MK (2013) Experimental thermal conductivity of ethylene glycol and water mixture based low volume concentration of Al2O3 and CuO nanofluids. Int Commun Heat Mass Transf 41:41–46. https://doi.org/10.1016/j.icheatmasstransfer.2012.11.004
Perkins RA, Roder HM, Nieto de Castro CA (1991) A high-temperature transient hot-wire thermal conductivity apparatus for fluids. J Res Natl Inst Stand Technol 96(3):247–269. https://doi.org/10.6028/jres.096.014
Azarfar S, Movahedirad S, Sarbanha AA et al (2016) Low cost and new design of transient hot-wire technique for the thermal conductivity measurement of fluids. Appl Therm Eng 105:142–150. https://doi.org/10.1016/j.applthermaleng.2016.05.138
Lee S, Choi SU-S, Li S, Eastman JA (1999) Measuring Thermal Conductivity of Fluids Containing Oxide Nanoparticles. J Heat Transf 121(2):280–289. https://doi.org/10.1115/1.2825978
Healy JJ, de Groot JJ, Kestin J (1976) The theory of the transient hot-wire method for measuring thermal conductivity. Phys B+C 82:392–408. https://doi.org/10.1016/0378-4363(76)90203-5
Lee JH, Hwang KS, Jang SP et al (2008) Effective viscosities and thermal conductivities of aqueous nanofluids containing low volume concentrations of Al2O3 nanoparticles. Int J Heat Mass Transf 51:2651–2656. https://doi.org/10.1016/j.ijheatmasstransfer.2007.10.026
Saidur R, Leong KY, Mohammad HA (2011) A review on applications and challenges of nanofluids. Renew Sust Energ Rev 15:1646–1668. https://doi.org/10.1016/j.rser.2010.11.035
Sundar LS, Singh MK, Sousa ACM (2013) Investigation of thermal conductivity and viscosity of Fe3O4 nanofluid for heat transfer applications. Int Commun Heat Mass Transf 44:7–14. https://doi.org/10.1016/j.icheatmasstransfer.2013.02.014
Viswanath DS, Ghosh TK, Prasad DHL et al (2007) Viscosity of liquids: Theory, estimation, experiment, and data, 1st edn. Springer Netherlands, Dordrecht. https://doi.org/10.1007/978-1-4020-5482-2
Pak BC, Cho YI (1998) Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp Heat Transf 11:151–170. https://doi.org/10.1080/08916159808946559
Leong KY, Saidur R, Mahlia TMI, Yau YH (2012) Modeling of shell and tube heat recovery exchanger operated with nanofluid based coolants. Int J Heat Mass Transf 55:808–816. https://doi.org/10.1016/j.ijheatmasstransfer.2011.10.027
Leong KY, Saidur R, Khairulmaini M et al (2012) Heat transfer and entropy analysis of three different types of heat exchangers operated with nanofluids. Int Commun Heat Mass Transf 39:838–843. https://doi.org/10.1016/j.icheatmasstransfer.2012.04.003
Abu-Nada E, Oztop HF (2009) Effects of inclination angle on natural convection in enclosures filled with Cu-water nanofluid. Int J Heat Fluid Flow 30:669–678. https://doi.org/10.1016/j.ijheatfluidflow.2009.02.001
Duangthongsuk W, Wongwises S (2009) Heat transfer enhancement and pressure drop characteristics of TiO2-water nanofluid in a double-tube counter flow heat exchanger. Int J Heat Mass Transf 52:2059–2067. https://doi.org/10.1016/j.ijheatmasstransfer.2008.10.023
Maxwell JC (2010) A treatise on electricity and magnetism. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511709340
Hamilton R, Crosser O (1962) Thermal conductivity of heterogeneous two-component systems. Ind Eng Chem Fundam 1(3):187–191. https://doi.org/10.1021/i160003a005
Bruggeman DAG (1937) Berechnung Verschiedener Physikalischer Konstanten von Heterogenen Substanzen, I. Dielektrizitatskonstanten und Leitfahigkeiten der Mischkorper aus Isotropen Substanzen. Ann Phys 416:636–664. https://doi.org/10.1002/andp.19354160705
Corcione M (2011) Empirical correlating equations for predicting the effective thermal conductivity and dynamic viscosity of nanofluids. Energy Convers Manag 52:789–793. https://doi.org/10.1016/j.enconman.2010.06.072
Patel HE, Sundararajan T, Das SK (2008) A cell model approach for thermal conductivity of nanofluids. J Nanopart Res 10:87–97. https://doi.org/10.1007/s11051-007-9236-4
Koo J, Kleinstreuer C (2004) A new thermal conductivity model for nanofluids. J Nanopart Res 6:577–588. https://doi.org/10.1007/s11051-004-3170-5
Xuan Y, Li Q, Hu W (2003) Aggregation structure and thermal conductivity of nanofluids. AICHE J 49:1038–1043. https://doi.org/10.1002/aic.690490420
Einstein A (1906) Eine neue Bestimmung der Molekul-dimensionen. Ann Phys 324:289–306. https://doi.org/10.1002/andp.19063240204
Batchelor GK (1977) The effect of Brownian motion on the bulk stress in a suspension of spherical particles. J Fluid Mech 83:97–117. https://doi.org/10.1017/S0022112077001062
Birkman HC (1952) The viscosity of concentrated suspensions and solution. J Chem Phys 20:571. https://doi.org/10.1063/1.1700493
Linstrom PJ, Mallard WG (2014) NIST Chemistry webBook. NIST Standard Reference Database Number 69, Washington D.C.
Evans W, Fish J, Keblinski P (2006) Role of Brownian motion hydrodynamics on nanofluid thermal conductivity. Appl Phys Lett 88:093116. https://doi.org/10.1063/1.2179118
Gupta A, Kumar R (2007) Role of Brownian motion on the thermal conductivity enhancement of nanofluids. Appl Phys Lett 91:223102. https://doi.org/10.1063/1.2816903
Jang SP, Choi SUS (2004) Role of Brownian motion in the enhanced thermal conductivity of nanofluids. Appl Phys Lett 84:4316. https://doi.org/10.1063/1.1756684
Shukla RK, Dhir VK (2008) Effect of Brownian Motion on Thermal Conductivity of Nanofluids. J Heat Transf 130:042406. https://doi.org/10.1115/1.2818768
Shima PD, Philip J, Raj B (2009) Role of microconvection induced by Brownian motion of nanoparticles in the enhanced thermal conductivity of stable nanofluids. Appl Phys Lett 94:223101. https://doi.org/10.1063/1.3147855
Teng T, Hung Y, Teng T et al (2010) The effect of alumina / water nano fl uid particle size on thermal conductivity. Appl Therm Eng 30:2213–2218. https://doi.org/10.1016/j.applthermaleng.2010.05.036
Teng T-P, Hung Y-H (2014) Estimation and experimental study of the density and specific heat for alumina nanofluid. J Exp Nanosci 9:707–718. https://doi.org/10.1080/17458080.2012.696219
Mariano A, Pastoriza-Gallego MJ, Lugo L et al (2013) Thermal conductivity, rheological behaviour and density of non-Newtonian ethylene glycol-based SnO2 nanofluids. Fluid Phase Equilib 337:119–124. https://doi.org/10.1016/j.fluid.2012.09.029
Taylor JR (1997) An Introduction to Error Analysis: The study of uncertainties in physical measurements, 2nd edn. University Science Books, Sausalito
Longo GA, Zilio C (2011) Experimental measurement of thermophysical properties of oxide-water nano-fluids down to ice-point. Exp Thermal Fluid Sci 35:1313–1324. https://doi.org/10.1016/j.expthermflusci.2011.04.019
Wen D, Ding Y (2005) Formulation of nanofluids for natural convective heat transfer applications. Int J Heat Fluid Flow 26:855–864. https://doi.org/10.1016/j.ijheatfluidflow.2005.10.005
Acknowledgements
The first author is thankful to the Ministry of Human Resource and Development (MHRD), Govt. Of India for providing fellowship and funding the on-going research on nanofluids under Annual Grant No. MED/AnnualGrant/4127/16-17. The first author would also like to thank Vinay Kumar, Electronics Engg, Dept., SVNIT, for his views on circuit formation for Transient hot-wire method and Sanjay Krishna, Chemical Engg. Dept., SVNIT for zeta potential measurements. The authors are grateful to the Department of Mechanical Engineering, SVNIT, Surat for all the support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
1.1 Uncertainty in thermal conductivity measurement
By replacing ΔT by Resistance terms in Eq. (12), we get
where ΔR = Rw-Ri.
The uncertainty for the term (Rw-Ri) can be computed by adding the uncertainty of the individual resistance and the uncertainty in thermal conductivity can be determined as shown in Eq. (14):
Therefore, total uncertainty in k is as follows:
The uncertainty in thermal conductivity measurement is ±0.01661 W/mK (±2.6%).
1.2 Uncertainty in density measurement
The density of any substance is given by:
Since mass and volume are two independent measurements, it is possible to apply the quadrature equation for predicting the uncertainty [65]. The equation is as follows:
Substituting the above values in Eq. (16), the uncertainty in density measurement was found to be
The uncertainty in density measurement is ±0.0177 g/cm3 or ± 17.7 kg/m3 (±1.7%).
C. Uncertainty in Viscosity measurement.
The dynamic viscosity (μ) is given by:
The uncertainty in viscosity measurement is as follows:
Before determining the uncertainty in dynamic viscosity measurement using Eq. (18), it is important to determine the partial uncertainties for density and kinematic viscosity measurement.
The kinematic viscosity equation is given by:
The uncertainty in kinematic viscosity measurement is as follows:
Therefore, we get δν = 0.00106 cSt since δt = 1 s.
Now, uncertainty in Dynamic viscosity can be obtained by:
Similarly,
Substituting in Eq. (18), δμ was found to be:
The uncertainty in dynamic viscosity measurement was found to be ±1.377×10−5 Pa.s or ± 0.0137 cP (±1.5%).
Rights and permissions
About this article
Cite this article
Nair, V., Parekh, A.D. & Tailor, P.R. Experimental investigation of thermophysical properties of R718 based nanofluids at low temperatures. Heat Mass Transfer 55, 2769–2784 (2019). https://doi.org/10.1007/s00231-019-02624-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-019-02624-y