Abstract
Objectives
To validate a deep learning (DL) algorithm for measurement of skeletal muscular index (SMI) and prediction of overall survival in oncology populations.
Methods
A retrospective single-center observational study included patients with metastatic renal cell carcinoma between 2007 and 2019. A set of 37 patients was used for technical validation of the algorithm, comparing manual vs DL-based evaluations. Segmentations were compared using mean Dice similarity coefficient (DSC), SMI using concordance correlation coefficient (CCC) and Bland-Altman plots. Overall survivals (OS) were compared using log-rank (Kaplan-Meier) and Mann-Whitney tests. Generalizability of the prognostic value was tested in an independent validation population (N = 87).
Results
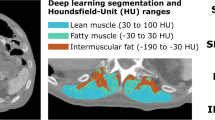
Differences between two manual segmentations (DSC = 0.91, CCC = 0.98 for areas) or manual vs. automated segmentation (DSC = 0.90, CCC = 0.98 for areas, CCC = 0.97 for SMI) had the same order of magnitude. Bland-Altman plots showed a mean difference of −3.33 cm2 [95%CI: −15.98, 9.1] between two manual segmentations, and −3.28 cm2 [95% CI: −14.77, 8.21] for manual vs. automated segmentations. With each method, 20/37 (56%) patients were classified as sarcopenic. Sarcopenic vs. non-sarcopenic groups had statistically different survival curves with median OS of 6.0 vs. 12.5 (p = 0.008) and 6.0 vs. 13.9 (p = 0.014) months respectively for manual and DL methods. In the independent validation population, sarcopenic patients according to DL had a lower OS (10.7 vs. 17.3 months, p = 0.033).
Conclusion
A DL algorithm allowed accurate estimation of SMI compared to manual reference standard. The DL-calculated SMI demonstrated a prognostic value in terms of OS.
Key Points
• A deep learning algorithm allows accurate estimation of skeletal muscle index compared to a manual reference standard with a concordance correlation coefficient of 0.97.
• Sarcopenic patients according to SMI thresholds after segmentation by the deep learning algorithm had statistically significantly lower overall survival compared to non-sarcopenic patients.




Similar content being viewed by others
Abbreviations
- BMI:
-
Body mass index
- CCC:
-
Concordance correlation coefficient
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- DL:
-
Deep learning
- DSC:
-
Dice similarity coefficient
- ECOG:
-
Eastern Cooperative Oncology Group
- FCN:
-
Fully convolutional network
- mRCC:
-
Metastatic renal cell carcinoma
- OS:
-
Overall survival
- RCC:
-
Renal cell carcinoma
- RMSE:
-
Root mean square error
- SD:
-
Standard deviation
- SMI:
-
Skeletal muscular index
References
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31. https://doi.org/10.1093/ageing/afy169
Shachar SS, Williams GR, Muss HB, Nishijima TF (2016)) Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer 57:58–67. https://doi.org/10.1016/j.ejca.2015.12.030
Auclin E, Bourillon C, De Maio E et al (2017) Prediction of everolimus toxicity and prognostic value of skeletal muscle index in patients with metastatic renal cell carcinoma. Clin Genitourin Cancer 15:350–355. https://doi.org/10.1016/j.clgc.2017.01.022
O’Connor JPB, Aboagye EO, Adams JE et al (2017) Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 14:169–186. https://doi.org/10.1038/nrclinonc.2016.162
Blanc-Durand P, Schiratti J-B, Schutte K et al (2020) Abdominal musculature segmentation and surface prediction from CT using deep learning for sarcopenia assessment. Diagn Interv Imaging. https://doi.org/10.1016/j.diii.2020.04.011
Lassau N, Bousaid I, Chouzenoux E et al (2020) Three artificial intelligence data challenges based on CT and MRI. Diagn Interv Imaging 101:783–788. https://doi.org/10.1016/j.diii.2020.03.006
Dice LR (1945) Measures of the amount of ecologic association between species. Ecology 26:297–302. https://doi.org/10.2307/1932409
Nie Z, Xu J, Zhang S (2020) Analysis on DeepLabV3+ performance for automatic steel defects detection. ArXiv200404822 Cs
Nagarajan P, Warnell G, Stone P (2019) Deterministic implementations for reproducibility in deep reinforcement learning. ArXiv180905676 Cs
Goodpaster BH, Thaete FL, Kelley DE (2000) Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci 904:18–24. https://doi.org/10.1111/j.1749-6632.2000.tb06416.x
Mourtzakis M, Prado CMM, Lieffers JR et al (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33:997–1006. https://doi.org/10.1139/H08-075
Fearon K, Strasser F, Anker SD et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12:489–495. https://doi.org/10.1016/S1470-2045(10)70218-7
Yushkevich PA, Piven J, Hazlett HC et al (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31:1116–1128. https://doi.org/10.1016/j.neuroimage.2006.01.015
Martin L, Birdsell L, Macdonald N et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol Off J Am Soc Clin Oncol 31:1539–1547. https://doi.org/10.1200/JCO.2012.45.2722
Zijdenbos AP, Dawant BM, Margolin RA, Palmer AC (1994) Morphometric analysis of white matter lesions in MR images: method and validation. IEEE Trans Med Imaging 13:716–724. https://doi.org/10.1109/42.363096
Grenier B, Dubreuil M, Journois D (2000) Comparaison de deux méthodes de mesure d’une même grandeur : méthode de Bland et Altman. Ann Fr Anesth Reanim 19:128–135. https://doi.org/10.1016/S0750-7658(00)00109-X
Chai T, Draxler RR (2014) Root mean square error (RMSE) or mean absolute error (MAE)?– Arguments against avoiding RMSE in the literature. Geosci Model Dev 7:1247–1250. https://doi.org/10.5194/gmd-7-1247-2014
Brodersen KH, Ong CS, Stephan KE, Buhmann JM (2010) The balanced accuracy and its posterior distribution. In: 2010 20th International Conference on Pattern Recognition. pp 3121–3124
Mouracade P (2017) Key concepts of survival analysis: checking appropriateness. Prog Urol Soc Francaise Urol 27:331–333. https://doi.org/10.1016/j.purol.2017.03.012
Burns JE, Yao J, Chalhoub D et al (2020) A machine learning algorithm to estimate sarcopenia on abdominal CT. Acad Radiol 27:311–320. https://doi.org/10.1016/j.acra.2019.03.011
Hu P, Huo Y, Kong D, et al (2018) Automated characterization of body composition and frailty with clinically acquired CT. Comput Methods Clin Appl Musculoskelet Imaging 5th Int Workshop MSKI 2017 Held Conjunction MICCAI 2017 Quebec City QC Can Sept 10 2017 Revis Sel Pap MSKI Work 10734:25–35. https://doi.org/10.1007/978-3-319-74113-0_3
Castiglione J, Somasundaram E, Gilligan LA et al (2021) Automated segmentation of abdominal skeletal muscle on pediatric CT scans using deep learning. Radiol Artif Intell 3:e200130. https://doi.org/10.1148/ryai.2021200130
Shahedi M, Cool DW, Romagnoli C et al (2014) Spatially varying accuracy and reproducibility of prostate segmentation in magnetic resonance images using manual and semiautomated methods. Med Phys 41:113503. https://doi.org/10.1118/1.4899182
Hermoye L, Laamari-Azjal I, Cao Z et al (2005) Liver segmentation in living liver transplant donors: comparison of semiautomatic and manual methods. Radiology 234:171–178. https://doi.org/10.1148/radiol.2341031801
El-Bana S, Al-Kabbany A, Sharkas M (2020) A two-stage framework for automated malignant pulmonary nodule detection in CT scans. Diagnostics (Basel) 10:131. https://doi.org/10.3390/diagnostics10030131
Weston AD, Korfiatis P, Kline TL et al (2018) Automated abdominal segmentation of CT scans for body composition analysis using deep learning. Radiology 290:669–679. https://doi.org/10.1148/radiol.2018181432
Hashimoto F, Kakimoto A, Ota N et al (2019) Automated segmentation of 2D low-dose CT images of the psoas-major muscle using deep convolutional neural networks. Radiol Phys Technol 12:210–215. https://doi.org/10.1007/s12194-019-00512-y
Lee H, Troschel FM, Tajmir S et al (2017) Pixel-level deep segmentation: artificial intelligence quantifies muscle on computed tomography for body morphometric analysis. J Digit Imaging 30:487–498. https://doi.org/10.1007/s10278-017-9988-z
Park HJ, Shin Y, Park J et al (2020) Development and validation of a deep learning system for segmentation of abdominal muscle and fat on computed tomography. Korean J Radiol 21:88–100. https://doi.org/10.3348/kjr.2019.0470
Chen L-C, Papandreou G, Kokkinos I, et al (2017) DeepLab: semantic image segmentation with deep convolutional nets, atrous convolution, and fully connected CRFs. ArXiv160600915 Cs
Belharbi S, Chatelain C, Hérault R et al (2017) Spotting L3 slice in CT scans using deep convolutional network and transfer learning. Comput Biol Med 87:95–103. https://doi.org/10.1016/j.compbiomed.2017.05.018
Acknowledgements
The authors wish to thank Charles-André Cuenod (CA), Gilles Soulat (GS), Mehdi Bouaboula (MB), and Jonas Deidier (JD) from the Radiology Department of the Hôpital européen Georges Pompidou, Assistance Publique-Hôpitaux de Paris, Paris, France for their help in evaluating for the presence of artifacts precluding the use of the segmentation algorithm. Mathilde Parent (MP), Marie Bruneel (MB), Victoria Tortochot (VT) students from the Université de Paris, AP-HP, Hôpital européen Georges Pompidou, Department of Radiology, PARCC UMRS 970, INSERM, Paris, France.
Funding
This study has received funding in part by the French Government under the management of the Agence Nationale de la Recherche as part of the “Investissements d’avenir” program, reference ANR-19-P3IA-0001 (PRAIRIE 3IA Institute).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Laure Fournier, MD PhD.
Conflict of Interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and Biometry
Armelle Arnoux, PhD, has significant statistical expertise.
She works in the university of Paris, AP-HP, Hôpital européen Georges Pompidou, Department of Biostatistics, Informatics and Clinical Research Unit, INSERM CIC1418-EC Clinical Epidemiology team, Paris, France.
Informed Consent
Written informed consent was waived by the Institutional Review Board.
Ethical Approval
Institutional Review Board approval was obtained, which waived the need for written informed consent. Indeed, in our institutions, the patient gives a global written consent to reuse his/her data for research and education, on condition that an IRB reviews the protocol and its ethical compliance to French law.
The IRB approval has been submitted with the manuscript.
Study subjects or cohorts overlap
Previously to this study, we had developed our diagnostic algorithm in a training population from a data challenge, and you will find enclosed the pdf of the paper referencing the population (Lassau et al, Diagn Interv Imaging 2020), which was one among three data challenges. One of our validation population has been previously published in part, and you will also find the pdf (Auclin et al, Genitourin Cancer 2017). In this previous study, the authors used a semi-automated method to calculate skeletal muscle index, and its purpose was to determine whether sarcopenia was predictive of prognosis and toxicity for metastatic renal cell cancer patients under everolimus. We included 44 of the 124 patients from the initial study, because we needed to have access to the original CT images of patients.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roblot, V., Giret, Y., Mezghani, S. et al. Validation of a deep learning segmentation algorithm to quantify the skeletal muscle index and sarcopenia in metastatic renal carcinoma. Eur Radiol 32, 4728–4737 (2022). https://doi.org/10.1007/s00330-022-08579-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08579-9