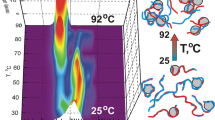
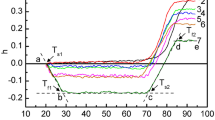
Abstract
TiO2 nanoparticles (NPs) are widely used in the environmental engineering, medicine, chemical and food industries due to their unique photocatalytic and biocidal properties. NPs may generate reactive oxygen species and, hence, have the toxic effect on the living cells via oxidative stress. An external UV irradiation may magnify the photocatalytic properties of TiO2 NPs. In this regard, we have analyzed the influence of TiO2 NPs on the conformation and thermal stability of native DNA in a buffer suspension without and under UV irradiation exploiting absorption spectroscopy and thermal denaturation in the range of 20–94 °C. The TiO2 NPs size distribution and polydispersity index were examined by dynamic light scattering (DLS) and confirmed by TEM. The DNA:TiO2 NPs assemblies were revealed and characterized by DLS and TEM. Upon heating the DNA suspension with TiO2 NPs from about 25 to 44 °C we have observed decreasing the hyperchromicity coefficient (h) on the DNA melting curves. That is explained by the intensive formation of the DNA:TiO2 NPs assemblies. We have revealed, that partial DNA disordering appears at initial contacts with NPs. DNA binding to TiO2 NPs is manifested in the change of the DNA melting temperature (Tm). We showed that the performed UV treatment of DNA during 3 h leads to partial unwinding of the biopolymer structure. The NPs injection to the biopolymer suspension induced the additional effect on the DNA thermal stability under UV irradiation. The performed analysis of the experimental data suggests that the nature of the impact of NPs on the biopolymer is complex.







Similar content being viewed by others
References
A.A. Dayem, M.K. Hossain, S.B. Lee, K. Kim, S.K. Saha, G.-M. Yang, H.Y. Choi, S.-G. Cho, Int. J. Mol. Sci. 18, 1 (2017)
C.L. de Dicastillo, M.G. Correa, F.B. Martínez, C. Streitt, M.J. Galotto, Antimicrobial effect of titanium dioxide nanoparticles, in Antimicrobial Resistance - A One Health Perspective. ed. by M. Mare, S.H.E. Lim, K. Lai, R. Cristina (IntechOpen, London, UK, 2020). https://doi.org/10.5772/intechopen.90891
K. McNamara, S.A. Tofail, Adv. Phys.: X 2(1), 54 (2017)
H. Wang, Y. Yao, G. Wu, Q. Sun, M. Wang, X. Chen, J. Wanget, A room temperature oxygen gas sensor based on hierarchical TiO2, IEEE International Conference on manipulation, manufacturing and measurement on the nanoscale (3M-NANO), 199 (2016)
Yu. Zhu, K. Yan, Z. Xu, J. Zhangz, J. Electrochem. Soc. 163(9), B526 (2016)
M. Ghadiry, M. Gholami, L. Choon Kong, C. Wu Yi, H. Ahmad, Y. Alias, Sensors 16, 39 (2016)
S. Jafari, B. Mahyad, H. Hashemzadeh, S. Janfaza, T. Gholikhani, L. Tayebi, Int J Nanomed. 14(150), 3447 (2020)
R. Vian, H. Salehi, M. Lapierre, F. Cuisinier, V. Cavaillès, S. Balme, Food Chem. 360, 130003 (2021)
Characterising the potential risks posed by engineered nanoparticles: a second UK government research report. Department of Environment, Food and Rural Affairs (DEFRA, London, UK, 2007)
EU nanotechnology R&D in the field of health and environmental impact of nanoparticles (Compiled by PilarAguar and José Juan Murcia Nicolás, Unit G4 Nano and Converging Sciences and Technologies European Commission, Research DG, 2008)
V. Aruoja, H.C. Dubourguier, K. Kasemets, A. Kahru, Sci. Total Environ. 407(4), 1461 (2009)
I. Velzeboer, A.J. Hendriks, A.M.J. Ragas, D. Van de Meent, Environ. Toxicol. Chem. 27(9), 1942 (2008)
C. Blaise, F. Gagne, J.F. Ferard, P. Eullaffroy, Environ. Toxicol. 23, 591 (2008)
S. Patel, P. Patel, S.R. Bakshi, Cytotechnology 69(2), 245 (2017)
R.P. Rastogi, Richa, A. Kumar, M.B. Tyagi, R.P. Sinha, J. Nucleic Acids 2010, 592980 (2010)
N. González, M. del Àngels Custal, D. Rodríguez, J.-R. Riba, E. Armelin, Mater. Res. 20(4), 1082 (2017)
T.A. Egerton, Molecules 19, 18192 (2014)
X. Zhang, F. Wang, B. Liu, E.Y. Kelly, M.R. Servos, J. Liu, Langmuir 30(3), 839 (2014)
K. Li, S. Du, S. Van Ginkel, Y. Chen, Atomic force microscopy study of the interaction of DNA and nanoparticles, in Nanomaterial, Advances in Experimental Medicine and Biology, vol. 811, ed. by D.G. Capco, Y. Chen (Springer, Dordrecht, 2014), pp.93–109
S. Patel, P. Patel, S.B. Undre, Sh.R. Pandya, M. Singh, S. Bakshi, J. Mol. Liq. 213, 304 (2016)
K.S. El-said, E.M. Ali, K. Kanehira, A. Taniguchi, J. Nanobiotechnol. 12, 48 (2014)
DNA Replication: From Old Principles to New Discoveries (Advances in Experimental Medicine and Biology Book 1042) 1st ed. 2017 Edition, Kindle Edition
V.A. Sorokin, V.A. Valeev, E.L. Usenko, V.V. Andrushchenko, Int. J. Biol. Macromol. 50(3), 854 (2012)
K. Higasi, H. Baba, A. Rembaum, Quantum Organic Chemistry (Interscience (A division John Wiley & Sons), New York, 1965)
E.L. Usenko, V.A. Valeev, AYu. Glamazda, V.A. Karachevtsev, J. Spectrosc. (2020). https://doi.org/10.1155/2020/8850214
M.V. Karachevtsev, O.S. Lytvyn, S.G. Stepanian, V.S. Leontiev, L. Adamowicz, V.A. Karachevtsev, J. Nanosci. Nanotech. 8(3), 1473 (2008)
C. Cascio, O. Geiss, F. Franchini, I. Ojea-Jimenez, F. Rossi, D. Gilliland, L. Calzolai, J. Anal. At. Spectrom 30, 1255 (2015)
I. de la Calle, M. Menta, M. Kleina, B. Maxit, F. Séby, SpectrochimicaActa Part B 147, 28 (2018)
B. Krause, T. Meyer, H. Sieg, C. Kästner, P. Reichardt, J. Tentschert, H. Jungnickel, I. Estrela-Lopis, A. Burele, S. Chevance, F. Gauffre, P. Jalili, J. Meijer, L. Boehmert, A. Braeuning, A.F. Thünemann, F. Emmerling, V. Fessard, P. Laux, A. Lampen, A. Luch, RSC Adv. 8, 14377 (2018)
T.G.F. Souza, V.S.T. Ciminelli, N.D.S. Mohallem, J. Phys.: Conf. Ser. 733, 012039 (2016)
Th. Dasri, P. Chaiyachate, S. Audtarat, P. Charee, A. Chingsungnoen, J. Met. Mater. Miner. 30(3), 30 (2020)
J. Kiefer, Effects of ultraviolet radiation on DNA, in Chromosomal Alterations: Methods, Results and Importance in Human Health. ed. by G. Obe, Vijayalaxmi (Springer, Berlin Heidelberg, 2007), pp.39–53
A.P. Sarapultsev, S.V. Rempel, J.V. Kuznetsova, G.P. Sarapultsev, J. Ural Med. Acad. Sci. (2016). https://doi.org/10.22138/2500-0918-2016-15-3-97-111
N.S. Leonenko, O.B. Leonenko, Innov. Biosyst. Bioeng. 4(2), 75 (2020)
H.M. Ali, H. Babar, T.R. Shah, M.U. Sajid, M.A. Qasim, S. Javed, Appl. Sci. 8(4), 587 (2018)
N.A. Kasyanenko, A.A. Andreeva, A.V. Baryshev, V.M. Bakulev, M.N. Likhodeeva, P.N. Vorontsov-Velyaminov, J. Phys. Chem. B 123, 9557 (2019)
Acknowledgements
Authors acknowledge financial support from National Academy of Sciences of Ukraine (Grant No. 0120U100157).
Funding
National Academy of Sciences of Ukraine, 0120U100157, Victor Karachevtsev, 0120U100157, Alexander Glamazda, 0120U100157, Evgeniya Usenko, 0120U100157, Vladimir Valeev.
Author information
Authors and Affiliations
Contributions
All authors discussed the results and commented on the manuscript. VV and EU carried out the spectroscopic measurements, thermal denaturation and analyzed data. AS and AL performed and analyzed the DLS data. SP performed TEM characterization. AG and VK planned and coordinated the project. EU, AG, and VK wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any commercial or associative interest that represents any conflict of interest in connection with the work submitted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Usenko, E., Glamazda, A., Valeev, V. et al. Effect of TiO2 nanoparticles on the thermal stability of native DNA under UV irradiation. Appl. Phys. A 128, 900 (2022). https://doi.org/10.1007/s00339-022-06043-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-06043-5