Abstract
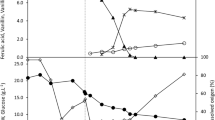
This study investigated the effects of transformation conditions such as initial pH, the initial concentration of glucose and yeast extract in the medium, and the separate addition of ferulic acid and vanillic acid, on the production of vanillin through an analysis of competing by-product formation by Amycolatopsis sp. ATCC 39116. The extent and nature of by-product formation and vanillin yield were affected by initial pH and different initial concentrations of glucose and yeast extract in the medium, with a high yield of vanillin and high cell density obtained at pH 8.0, 10 g/l glucose, and 8 g/l yeast extract. High concentrations of ferulic acid were found to negatively affect cell density. Additional supplementation of 100 mg/l vanillic acid, a metabolically linked by-product, was found to result in a high concentration of vanillin and guaiacol, an intermediate of vanillin. Via an analysis of the effect of these transformation conditions on competing by-product formation, high concentrations of ferulic acid were transformed with a molar yield to vanillin of 96.1 and 95.2 %, by Amycolatopsis sp. ATCC 39116 and Streptomyces V1, respectively, together with a minor accumulation of by-products. These are among the highest performance values reported in the literature to date for Streptomyces in batch cultures.





Similar content being viewed by others
References
Webster TM (1995) New perspectives on vanilla. Cereal Foods World 40(4):198–200
Priefert H, Rabenhorst J, Steinbuchel A (2001) Biotechnological production of vanillin. Appl Microbiol Biotechnol 56(3–4):296–314
Toms A, Wood JM (1970) The degradation of trans-ferulic acid by Pseudomonas acidovorans. Biochemistry 9(2):337–343
Ashengroph M, Nahvi I, Zarkesh-Esfahani H, Momenbeik F (2012) Conversion of isoeugenol to vanillin by Psychrobacter sp. strain CSW4. Appl Biochem Biotechnol 166(1):1–12
Ashengroph M, Nahvi I, Zarkesh-Esfahani H, Momenbeik F (2011) Candida galli strain PGO6: a novel isolated yeast strain capable of transformation of isoeugenol into vanillin and vanillic acid. Curr Microbiol 62(3):990–998
Ashengroph M, Nahvi I, Zarkesh-Esfahani H, Momenbeik F (2011) Pseudomonas resinovorans SPR1, a newly isolated strain with potential of transforming eugenol to vanillin and vanillic acid. New Biotechnol 28(6):656–664
Su F, Hua DL, Zhang ZB, Wang XY, Tang HZ, Tao F, Tai C, Wu QL, Wu G, Xu P (2011) Genome sequence of bacillus pumilus S-1, an efficient isoeugenol-utilizing producer for natural vanillin. J Bacteriol 193(22):6400–6401
Hua DL, Ma CQ, Song LF, Lin S, Zhang ZB, Deng ZX, Xu P (2007) Enhanced vanillin production from ferulic acid using adsorbent resin. Appl Microbiol Biotechnol 74(4):783–790
Wangrangsimagul N, Klinsakul K, Vangnai AS, Wongkongkatep J, Inprakhon P, Honda K, Ohtake H, Kato J, Pongtharangkul T (2012) Bioproduction of vanillin using an organic solvent-tolerant Brevibacillus agri 13. Appl Microbiol Biotechnol 93(2):555–563
Ghosh S, Sachan A, Sen SK, Mitra A (2007) Microbial transformation of ferulic acid to vanillic acid by Streptomyces sannanensis MTCC 6637. J Ind Microbiol Biotechnol 34(2):131–138
Narbad A, Gasson MJ (1998) Metabolism of ferulic acid via vanillin using a novel CoA-dependent pathway in a newly-isolated strain of Pseudomonas fluorescens. Microbiology 144(Pt 5):1397–1405
Bode HB, Muller R (2003) Possibility of bacterial recruitment of plant genes associated with the biosynthesis of secondary metabolites. Plant Physiol 132(3):1153–1161
Muheim A, Lerch K (1999) Towards a high-yield bioconversion of ferulic acid to vanillin. Appl Microbiol Biotechnol 51(4):456–461
Zhao LQ, Sun ZH, Zheng P, Zhu LL (2005) Biotransformation of isoeugenol to vanillin by a novel strain of Bacillus fusiformis. Biotechnol Lett 27(19):1505–1509
Mathew S, Abraham TE, Sudheesh S (2007) Rapid conversion of ferulic acid to 4-vinyl guaiacol and vanillin metabolites by Debaryomyces hansenii. J Mol Catal B-Enzymatic 44(2):48–52
Mathew S, Abraham TE, Sudheesh S (2007) Rapid conversion of ferulic acid to 4-vinyl guaiacol and vanillin metabolites by Debaryomyces hansenii. J Mol Catal B Enzym 44(2):48–52
Karmakar B, Vohra RM, Nandanwar H, Sharma P, Gupta KG, Sobti RC (2000) Rapid degradation of ferulic acid via 4-vinylguaiacol and vanillin by a newly isolated strain of Bacillus coagulans. J Biotechnol 80:195–202
Fleige C, Hansen G, Kroll J, Steinbuchel A (2013) Investigation of the Amycolatopsis sp. strain ATCC 39116 vanillin dehydrogenase and its impact on the biotechnical production of vanillin. Appl Environ Microbiol 79(1):81–90
Sutherland JB, Crawford DL, Pometto AL III (1983) Metabolism of cinnamic, p-coumaric, and ferulic acids by Streptomyces setonii. Can J Microbiol 29(10):1253–1257
Li T, Rosazza JP (2000) Biocatalytic synthesis of vanillin. Appl Environ Microbiol 66(2):684–687
Yoon SH, Lee EG, Das A, Lee SH, Li C, Ryu HK, Choi MS, Seo WT, Kim SW (2007) Enhanced vanillin production from recombinant E-coli using NTG mutagenesis and adsorbent resin. Biotechnol Prog 23(5):1143–1148
Gao F, Daugulis AJ (2009) Bioproduction of the aroma compound 2-phenylethanol in a solid-liquid two-phase partitioning bioreactor system by kluyveromyces marxianus. Biotechnol Bioeng 104(2):332–339
Acknowledgments
The Project was supported by Social Development and Scientific and Technological Plan in Shaanxi Province of China (Program No. 693102), Science and technology projects in Xi’an of China (No.694180) and the Fundamental Research Funds for the Central Universities of China (No.GK201003002). Many thanks to Julian Dafoe and Eric Peterson for their support during this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, Xk., Daugulis, A.J. Effect of bioconversion conditions on vanillin production by Amycolatopsis sp. ATCC 39116 through an analysis of competing by-product formation. Bioprocess Biosyst Eng 37, 891–899 (2014). https://doi.org/10.1007/s00449-013-1060-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-013-1060-x