Abstract
A non-enzymatic electrochemical method was developed for sensing glucose by using a glassy carbon electrode modified with 3-aminophenylboronic acid (APBA) immobilized on polyethyleneimine (PEI)-coated gold nanoparticles. The modified electrode was characterized by TEM, zeta potential measurements and UV-Vis spectroscopy. Its analytical performance was evaluated in pH 9 solution by potentiometry. The respective calibration plot, established at open circuit potential (vs. Ag/AgCl) covers the 0.5–50 mM glucose concentration range, which makes it suitable for blood glucose assays. The detection limit is 0.025 mM, and no interference is caused by ascorbic acid, dopamine, and uric acid. Effects of other carbohydrates such as fructose, galactose and saccharose were also investigated. The electrode was used to determine glucose in human serum samples and the results agreed well with those obtained with commercial amperometric enzymatic sensors.

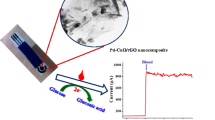
Schematic of the preparation of polyethyleneimine (PEI) coated gold nanoparticles modified with 3-aminophenylboronic acid for use in non-enzymatic potentiometric determinaton of glucose based on molecular recognition







Similar content being viewed by others
References
Jeffrey DN, Anthony PFT (2005) Home blood glucose biosensors: a commercial perspective. Biosens Bioelectron 20:2435–2453
Xiaomei C, Genghuang W, Zhixiong C, Munetaka O, Xi C (2014) Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim Acta 181:689–705
Tian K, Prestgard M, Tiwari A (2014) A review of recent advances in nonenzymatic glucose sensors. Mater Sci Eng C 41:100–118
Wang Q, Cui X, Chen J, Zheng X, Liu C, Xue T, Wang H, Jin Z, Qiao L, Zheng W (2012) Well-dispersed palladium nanoparticles on graphene oxide as a non-enzymatic glucose sensor. RSC Adv 2:6245–6249
Mallesha M, Manjunatha R, Suresh GS, Melo JS, D’Souza SF, Venkatesha TV (2012) Direct electrochemical non-enzymatic assay of glucose using functionalized graphene. J Solid State Electrochem 16:2675–2681
Kun T, Megan P, Ashutosh T (2014) A review of recent advances in nonenzymatic glucose sensors. Mater Sci Eng C 41:100–118
Torun O, Dudak FC, Bas D, Tamer U, Boyacı IH (2009) Thermodynamic analysis of the interaction between 3-aminophenylboronic acid and monosaccharides for development of biosensor. Sensor Actuat B-Chem 140:597–602
Ciftci H, Tamer U, Teker MS, Pekmez NO (2013) An enzyme free potentiometric detection of glucose based on a conducting polymer poly (3-aminophenyl boronic acid-co-3-octylthiophene). Electrochim Acta 90:358–365
Mader HS, Wolfbeis OS (2008) Boronic acid based probes for microdetermination of saccharides and glycosylated biomolecules. Microchim Acta 162:1–34
Nguyen QH, Ali M, Neumann R, Ensinger W (2012) Saccharide/glycoprotein recognition inside synthetic ion channels modified with boronic acid. Sensor Actuat B-Chem 162:216–222
Steiner MS, Duerkop A, Wolfbeis OS (2011) Optical methods for sensing glucose. Chem Soc Rev 40:4805–4839
Tsukagoshi K, Matsumoto K, Ueno F, Noda K, Nakajima R, Araki K (2006) Molecular recognition of mono- and disaccharides through interaction with p-iodophenylboronic acid in capillary electrophoresis with a chemiluminescence detection system. J Chromatogr A 1123:106–112
Wen S, Zheng F, Shen M, Shi X (2013) Synthesis of polyethyleneimine-stabilized gold nanoparticles for colorimetric sensing of heparin. Colloid Surf A 419:80–86
Nikov RG, Nikolov AS, Nedyalkov NN, Dimitrov IG, Atanasov PA, Alexandrov MT (2012) Stability of contamination-free gold and silver nanoparticles produced by nanosecond laser ablation of solid targets in water. Appl Surf Sci 258:9318–9322
Lee HJ, Lee SG, Oh EJ, Chung HY, Han SI, Kim EJ, Seo SY, Ghim HD, Yeum JH, Choi JH (2011) Antimicrobial polyethyleneimine-silver nanoparticles in a stable colloidal dispersion. Colloid Surf B 88:505–511
Jahan S, Mansoor F, Kanwal S (2014) Polymers effects on synthesis of AuNPs, and Au/Ag nanoalloys: indirectly generated AuNPs and versatile sensing applications including anti-leukemic agent. Biosens Bioelectron 53:51–57
Shang L, Jin L, Guo S, Zhai J, Dong S (2010) A facile and controllable strategy to synthesize Au-Ag alloy nanoparticles within polyelectrolyte multilayer nanoreactors upon thermal reduction. Langmuir 26:6713–6719
Frasca S, Rojas O, Salewski, Neumann B, Stiba K, Weidinger I, Tiersch B, Leimkühler S, Koetz J, Wollenberger U (2012) Human sulfite oxidase electrochemistry on gold nanoparticles modified electrode. Bioelectrochem 87:33–41
Cebrian V, Martin-Saavedra F, Yagüe C, Arruebo M, Santamaria J, Vilaboa N (2011) Size-dependent transfection efficiency of PEI-coated gold nanoparticles. Acta Biomater 7:3645–3655
Lou L, Yu K, Zhang Z, Huang R, Wang Y, Zhu Z (2012) Facile methods for synthesis of core-shell structured and heterostructured Fe3O4@Au nanocomposites. Appl Surf Sci 258:8521–8526
Thete A, Rojas O, Neumeyer D, Koetz J, Dujuardin E (2013) Ionic liquid-assisted morphosynthesis of gold nanorods using polyethyleneimine-capped seeds. RSC Adv 3:14294–14298
Kim EJ, Yeum JH, Choi JH (2014) Effects of polymeric stabilizers on the synthesis of gold nanoparticles. J Mater Sci Technol 30(2):107–111
Liz-Marzan LM (2004) Nanometals: formation and color. Mater Today 7:26–31
Liu A, Ren Q, Xu T, Yuan M, Tang W (2012) Morphology-controllable gold nanostructures on phosphorus doped diamond-like carbon surfaces and their electrocatalysis for glucose oxidation. Sensor Actuat B-Chem 162:135–142
Feng D, Wang F, Chen ZL (2009) Electrochemical glucose sensor based on onestep construction of gold nanoparticle-chitosan composite film. Sensor Actuat B-Chem 138:539–544
Çiftçi H, Tamer U (2012) Functional gold nanorod particles on conducting polymer poly(3-octylthiophene) as non-enzymatic glucose sensor. React Funct Polym 72:127–132
Bo X, Bai J, Yang L, Guo L (2011) The nanocomposite of PtPd nanoparticles/onion-likemesoporous carbon vesicle for nonenzymatic amperometric sensing of glucose. Sensor Actuat B-Chem 157:662–668
Gao H, Xiao F, Ching C, Duan H (2011) One-step electrochemical synthesis of PtNinanoparticle-graphene nanocomposites for nonenzymatic amperometric glucose detection. ACS Appl Mater Interfaces 3:3049–3057
Li M, Bo X, Mu Z, Zhang Y, Guo L (2014) Electrodeposition of nickel oxide and platinum nanoparticles on electrochemically reduced graphene oxide film as a nonenzymatic glucose sensor. Sensor Actuat B-Chem 192:261–268
Zhang Y, Wang Y, Jia J, Wang J (2012) Nonenzymatic glucose sensor based on graphene oxide and electrospun NiO nanofibers. Sensor Actuat B-Chem 171–172:580–587
Li J, Liu L, Wang P, Zheng J (2014) Potentiometric detection of saccharides based on highly ordered poly(aniline boronic acid) nanotubes. Electrochim Acta 121:369–375
Manesh KM, Santhosh P, Gopalan A, Lee KP (2007) Electrospun poly(vinylidene xuoride)/poly(aminophenylboronic acid) composite nanofibrous membrane as a novel glucose sensor. Anal Biochem 360:189–195
Alexeev L, Sharma AC, Goponenko AV, Das S, Lednev IK, Wilcox CS, Finegold DN, Asher SA (2003) High ionic strength glucose- sensing photonic crystal. Anal Chem 75:2316–2323
Mei H, Wu W, Yu B, Li Y, Wu H, Wang S, Xia Q (2015) Non-enzymatic sensing of glucose at neutral pH values using a glassy carbon electrode modified with carbon supported Co@Pt core-shell nanoparticles. Microchim Acta 182:1869–1875
Acknowledgments
The authors gratefully acknowledge the financial support from Kırıkkale University Research Fund through Grant no.: 2015/48.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests
Electronic supplementary material
ESM 1
(DOCX 157 kb)
Rights and permissions
About this article
Cite this article
Çiftçi, H., Alver, E., Çelik, F. et al. Non-enzymatic sensing of glucose using a glassy carbon electrode modified with gold nanoparticles coated with polyethyleneimine and 3-aminophenylboronic acid. Microchim Acta 183, 1479–1486 (2016). https://doi.org/10.1007/s00604-016-1782-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1782-y