Abstract
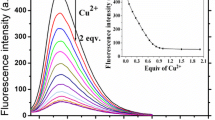
A fluorometric assay is described for sulfide ions determination. It is based on the finding that the oxidation of the non-fluorescent substrate thiamine (TH) by Cu(II) in basic solution to form fluorescent thiochrome is inhibited by sulfide ions. This results in a decrease in fluorescence intensity which is proportional to the concentration of sulfide ions. Under the optimized conditions, the decrease in fluorescence, best measured at excitation/emission wavelengths of 370/440 nm, decreases linearly in the 0.03 to 2.5 μM sulfide ions concentration range. The detection limit is 20 nM. The method shows excellent selectivity over many potentially interfering ions and has been successfully applied to the determination of sulfide ions in spiked tap water, lake water and the synthetic wastewater samples. The method is time-saving and environmentally friendly, and in our perception shows a great potential in water quality inspection and environmental monitoring.

A fluorescent assay for sulfide ions detection is proposed based on its inhibitory effect on the oxidation of thiamine by Cu(II) ions.




Similar content being viewed by others
References
Ni PJ, Sun YJ, Dai HC, Hu JT, Jiang S, Wang YL, Li Z, Li Z (2015) Colorimetric detection of sulfide ions in water samples based on the in situ formation of Ag2S nanoparticles. Sensors Actuators B Chem 220:210–215
Liao H, Hu LZ, Zhang YZ, Yu XR, Liu YL, Li R (2018) A highly selective colorimetric sulfide assay based on the inhibition of the peroxidase-like activity of copper nanoclusters. Microchim Acta 185:143
Shanmugaraj K, Ilanchelian M (2016) Colorimetric determination of sulfide using chitosan-capped silver nanoparticles. Microchim Acta 183:1721–1728
Weng H, Yan B (2017) A Eu(III) doped metal-organic framework conjugated with fluorescein-labeled single-stranded DNA for detection of cu(II) and sulfide. Anal Chim Acta 988:89–95
Balasubramanian S, Pugalenthi V (2000) A comparative study of the determination of sulphide in tannery waste water by ion selective electrode (ISE) and iodimetry. Water Res 34:4201–4206
Rembisz Z, Bzdurska D, Obiedzinska J, Martinez-Manez R, Zakrzewski R (2015) A derivatization approach using pyrylium salts for the sensitive and simple determination of sulfide in spring water by high performance liquid chromatography. J Chromatogr A 1407:184–192
Daunoravicius Z, Padarauskas A (2002) Capillary electrophoretic determination of thiosulfate, sulfide and sulfite using in-capillary derivatization with iodine. Electrophoresis 23:2439–2444
Su YZ, Yang SY, Liu WP, Qiao L, Yan J, Liu YJ, Zhang SS, Fang YP (2017) Visible light photoelectrochemical sulfide sensor based the use of TiO2 nanotube arrays loaded with Cu2O. Microchim Acta 184:4065–4072
Liu BB, Han SQ (2016) Determination of trace hydrogen sulfide by using the permanganate induced chemiluminescence of carbon dots. Microchim Acta 183:3087–3092
Jiang HE, Liu Y, Luo WF, Wang YJ, Tang XL, Dou W, Cui YM, Liu WS (2018) A resumable two-photon fluorescent probe for Cu2+ and S2− based on magnetic silica core-shell Fe3O4@SiO2 nanoparticles and its application in bioimaging. Anal Chim Acta 104:91–99
Na WD, Qu ZY, Chen XQ, Su XG (2018) A turn-on fluorescent probe for sensitive detection of sulfide anions and ascorbic acid by using sulfanilic acid and glutathione functionalized graphene quantum dots. Sensors Actuators B Chem 256:48–54
Zhang N, Si YM, Sun ZZ, Chen LJ, Li R, Qiao YC, Wang H (2014) Rapid, selective, and ultrasensitive fluorimetric analysis of mercury and copper levels in blood using bimetallic gold-silver nanoclusters with “silver effect”-enhanced red fluorescence. Anal Chem 86:11714–11721
Fan C, Lv XX, Liu FJ, Feng LP, Liu M, Cai YY, Liu H, Wang JY, Yang YL, Wang H (2018) Silver nanoclusters encapsulated into metal-organic frameworks with enhanced fluorescence and specific ion accumulation toward the microdot array-based fluorimetric analysis of copper in blood. ACS Sens 3:441–450
Feng LP, Sun ZZ, Liu H, Liu M, Jiang Y, Fan C, Cai YY, Zhang S, Xu JH, Wang H (2017) Silver nanoclusters with enhanced fluorescence and specific ion recognition capability triggered by alcohol solvents: a highly selective fluorimetric strategy for detecting iodide ions in urine. Chem Commun 53:3466–3469
Lippert AR, New EJ, Chang CJ (2011) Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J Am Chem Soc 133:10078–10080
Xuan WM, Sheng CQ, Cao YT, He WH, Wang W (2012) Fluorescent probes for the detection of hydrogen sulfide in biological systems. Angew Chem Int Ed 51:2282–2284
Liu CR, Pan J, Li S, Zhao Y, Wu LY, Berkman CE, Whorton AR, Xian M (2011) Capture and visualization of hydrogen sulfide by a fluorescent probe. Angew Chem Int Ed 50:10327–10329
Xu Z, Xu L, Zhou J, Xu YF, Zhu WP, Qian XH (2012) A highly selective fluorescent probe for fast detection of hydrogen sulfide in aqueous solution and living cells. Chem Commun 48:10871–10873
Li H (2018) A regenerated "turn on" fluorescent probe for sulfide detection in live cells and read samples based on dihydroxyhemicyanine-Cu2+ dye. Anal Chim Acta 1010:69–75
Zheng FY, Wen M, Zeng F, Wu SZ (2013) A robust, water-soluble and low cytotoxic fluorescent probe for sulfide anion achieved through incorporation of betaine. Sensors Actuators B Chem 188:1012–1018
Bai XL, Xu SY, Wang LY (2018) Full-range pH stable Au-clusters in nanogel for confinement-enhanced emission and improved sulfide sensing in living cells. Anal Chem 90:3270–3275
Yu NX, Peng HL, Xiong H, Wu XQ, Wang XY, Li YB, Chen LX (2015) Graphene quantum dots combined with copper(II) ions as a fluorescent probe for turn-on detection of sulfide ions. Microchim Acta 182:2139–2146
Tan HL, Li Q, Zhou ZC, Ma CJ, Song YH, Xu FG, Wang L (2015) A sensitive fluorescent assay for thiamine based on metal-organic frameworks with intrinsic peroxidase-like activity. Anal Chim Acta 856:90–95
Perez-Ruiz T, Martinez-Lozano C, Tomas V, Ibarra I (1992) Flow injection fluorimetric determination of thiamine and copper based on the formation of thiochrome. Talanta 39:907–911
Yang XF, Wang LP, Xu HM, Zhao ML (2009) A fluorescein-based fluorogenic and chromogenic chemodosimeter for the sensitive detection of sulfide in aqueous solution. Anal Chim Acta 631:91–95
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21705056), the Natural Science Foundation of Shandong Province (ZR2017MB022, ZR2018BB057 and ZR2018 PB009) and the start-up funding from University of Jinan (511-1009408, 511-1009424).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 3398 kb)
Rights and permissions
About this article
Cite this article
Ni, P., Chen, C., Jiang, Y. et al. Fluorometric determination of sulfide ions via its inhibitory effect on the oxidation of thiamine by Cu(II) ions. Microchim Acta 185, 362 (2018). https://doi.org/10.1007/s00604-018-2906-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2906-3