Abstract.
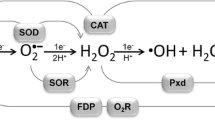
Numerous biological systems involve reaction with dioxygen in the absence of readily accessible spectroscopic signals. We have begun to develop a set of "generic" strategies that will allow us to probe the mechanisms of dioxygen activation. In particular, we wish to understand the nature of the dioxygen binding step, the degree to which electron transfer to dioxygen is rate limiting, whether reactive species accumulate during turnover and, finally, whether proton and electron transfer to dioxygen occur as coupled processes. Our strategy will be introduced for an enzyme system that uses only an organic cofactor in dioxygen activation (glucose oxidase). Two key features emerge from studies of glucose oxidase: (1) that formation of the superoxide anion is a major rate-limiting step and (2) that electrostatic stabilization of the superoxide anion plays a key role in catalysis. Similar themes emerge when our protocols are applied to enzymes containing both an active site metal center and an organic cofactor. Finally, enzymes that rely solely on metal centers for substrate functionalization will be discussed. In no instance, thus far, has evidence been found for a direct coupling of proton to electron transfer in the reductive activation of dioxygen.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Klinman, J. Life as aerobes: are there simple rules for activation of dioxygen by enzymes?. J. Biol. Inorg. Chem. 6, 1–13 (2001). https://doi.org/10.1007/s007750000172
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s007750000172