Abstract
Physical and chemical adsorption of CO2 on ZnO surfaces were studied by means of two different implementations of periodic density functional theory. Adsorption energies were computed and compared to values in the literature. In particular, it was found that the calculated equilibrium structure and internuclear distances are in agreement with previous work. CO2 adsorption was analyzed by inspection of the density of states and electron localization function. Valence bands, band gap and final states of adsorbed CO2 were investigated and the effect of atomic displacements analyzed. The partial density of states (PDOS) of chemical adsorption of CO2 on the ZnO(0001) surface show that the p orbitals of CO2 were mixed with the ZnO valence band state appearing at the top of the valence band and in regions of low-energy conduction band.

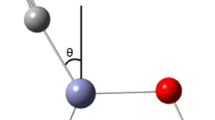
ELF analysis of bidentate and tridentate chemical adsorptions









Similar content being viewed by others
References
Abe K, Banno Y, Sasayama T, Koizumi K (2009) J Vac Sci Technol B 27:1652–1654. doi:10.1116/1.3089374
Beltran A, Andres J, Calatayud M, Martins JBL (2001) Theoretical study of ZnO (10(1)over-bar-0) and Cu/ZnO (10(1)over-bar-0) surfaces. Chem Phys Lett 338:224–230. doi:10.1016/S0009-2614(01)00238-X
Marana NL, Longo VM, Longo E, Martins JBL, Sambrano JR (2008) Electronic and structural properties of the (10(1)over-bar0) and (11(2)over-bar0) ZnO surfaces. J Phys Chem A 112:8958–8963
Tabatabaei J, Sakakini BH, Waugh KC (2006) On the mechanism of methanol synthesis and the water-gas shift reaction on ZnO. Catal Lett 110:77–84
Noguera C (2000) Polar oxide surfaces. J Phys Condens Matter 12:R367–R410
Noei H, Woll C, Mahler M, Wang YM (2011) Activation of carbon dioxide on ZnO nanoparticles studied by vibrational spectroscopy. J Phys Chem C 115:908–914. doi:10.1021/jp102751t
Martins JBL, Longo E, Salmon ODR, Espinoza VAA, Taft CA (2004) The interaction of H-2, CO, CO2, H2O and NH3 on ZnO surfaces: an Oniom study. Chem Phys Lett 400:481–486
Martins JBL, Longo E, Taft CA (1998) CO2 and NH3 interaction with ZnO surface: An AM1 study. Int J Quantum Chem 70:367–374
Martins JBL, Sambrano JR, Vasconcellos LAS, Longo E, Taft CA (2004) Theoretical analysis of the interaction of CO, CO2, and NH3, with ZnO. Quim Nova 27:10–16
Borodko Y, Somorjai GA (1999) Catalytic hydrogenation of carbon oxides—a 10-year perspective. Appl Catal A Gen 186:355–362
Fink K (2006) Ab initio cluster calculations on the electronic structure of oxygen vacancies at the polar ZnO(0001) surface and on the adsorption of H-2, CO, and CO2 at these sites. Phys Chem Chem Phys 8:1482–1489
Moreira NH, da Rosa AL, Frauenheim T (2009) Covalent functionalization of ZnO surfaces: a density functional tight binding study. Appl Phys Lett 94:193109
French SA, Sokol AA, Bromley ST, Catlow CRA, Rogers SC, King F, Sherwood P (2001) From CO2 to methanol by hybrid QM/MM embedding. Angew Chem Int Ed 40:4437–4440. doi:1433-7851/01/4023-4438
Chen WK, Zhang YF, Ding KN, Xu YJ, Li Y, Li JQ (2004) A density functional theory study of the adsorption of CO2 on a ZnO(10(1)over-bar0) surface. Chin J Struct Chem 23:337–341
Wang Y, Kovacik R, Meyer B, Kotsis K, Stodt D, Staemmler V, Qiu H, Traeger F, Langenberg D, Muhler M, Woll C (2007) CO2 activation by ZnO through the formation of an unusual tridentate surface carbonate. Angew Chem Int Ed 46:5624–5627. doi:10.1002/anie.200700564
Kotsis K, Stodt D, Staemmler V, Kovacik R, Meyer B, Traeger F, Langenberg D, Strunskus T, Kunat M, Woll C (2008) CO2 adlayers on the mixed terminated ZnO(10-10) surface studied by he atom scattering, photoelectron spectroscopy and ab initio electronic structure calculations. Z Phys Chem Int J Res Phys Chem Chem Phys 222:891–915
Davis R, Walsh JF, Muryn CA, Thornton G, Dhanak VR, Prince KC (1993) The orientation of formate and carbonate on Zno[10(1)over-Bar0]. Surf Sci 298:L196–L202
GutierrezSosa A, Crook S, Haq S, Lindsay R, Ludviksson A, Parker S, Campbell CT, Thornton G (1996) Influence of Cu overlayers on the interaction of CO and CO2 with ZnO(000(1)over-bar)-O. Faraday Discuss 105:355–368
Barteau MA (1993) Site requirements of reactions on oxide surfaces. J Vac Sci Technol A 11:2162–2168
Wang J, Burghaus U (2005) Adsorption of CO on the copper-precovered ZnO(0001) surface: a molecular-beam scattering study. J Chem Phys 123(18):184716
Wang J, Burghaus U (2005) Structure-activity relationship: the case of CO2 adsorption on H/Zn-ZnO(0001). Chem Phys Lett 403:42–46
Wang J, Burghaus U (2005) Adsorption dynamics of CO2 on Zn-ZnO(0001): a molecular beam study. J Chem Phys 122:044705
Göpel W, Bauer RS, Hansson G (1980) Ultraviolet photoemission studies of chemisorption and point defect formation on ZnO nonpolar surfaces. Surf Sci 99:138–156. doi:10.1016/0039-6028(80)90584-1
Hotan W, Göpel W, Haul R (1979) Interaction of CO2 and co with nonpolar zinc oxide surfaces. Surf Sci 83:162–180. doi:10.1016/0039-6028(79)90486-2
Gutierrez-Sosa A, Evans TM, Parker SC, Campbell CT, Thornton G (2002) Geometry of C1-3 oxygenates on ZnO(0001)-Zn. Surf Sci 497:239–246. doi:10.1016/S0039-6028(01)01645-4
Cheng WH, Kung HH (1982) Interaction of Co, Co2 and O2 with non-polar, stepped and polar Zn surfaces of Zno. Surf Sci 122:21–39
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev B 136:B864–B871
Zhang FY (2011) A possible new transition path for ZnO from B4 to B1. Physica B 406:3942–3946
Ashrafi A, Jagadish C (2007) Review of zincblende ZnO: stability of metastable ZnO phases. J Appl Phys 102:071101
Charifi Z, Baaziz H, Reshak AH (2007) Ab-initio investigation of structural, electronic and optical properties for three phases of ZnO compound. Phys Status Solidi B Basic Solid State Phys 244:3154–3167
Hill NA, Waghmare U (2000) First-principles study of strain-electronic interplay in ZnO: stress and temperature dependence of the piezoelectric constants. Phys Rev B 62:8802–8810
Schroer P, Kruger P, Pollmann J (1993) 1st-principles calculation of the electronic-structure of the wurtzite semiconductors Zno and Zns. Phys Rev B 47:6971–6980
Perdew JP (1991) Electronic structure of solids. Akademie, Berlin
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Mattsson AE, Armiento R, Schultz PA, Mattsson TR (2006) Nonequivalence of the generalized gradient approximations PBE and PW91. Phys Rev B 73:195123. doi:10.1103/PhysRevB.73.195123
Adllan AA, Corso AD (2011) Ultrasoft pseudopotentials and projector augmented-wave data sets: application to diatomic molecules. J Phys Condens Matter 23:425501. doi:10.1088/0953-8984/23/42/425501
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59:1758–1775
Swart M, Snijders JG (2003) Accuracy of geometries: influence of basis set, exchange–correlation potential, inclusion of core electrons, and relativistic corrections. Theor Chem Accounts 110:34–41. doi:10.1007/s00214-003-0443-5
Orita H, Itoh N, Inada Y (2004) All electron scalar relativistic calculations on adsorption of CO on Pt(1 1 1) with full-geometry optimization: a correct estimation for CO site-preference. Chem Phys Lett 384:271–276. doi:10.1016/j.cplett.2003.12.034
Kresse G, Furthmuller J (1996) Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6:15–50
Abrahams SC, Bernstein JL (1969) Remeasurement of the structure of hexagonal ZnO. Acta Cryst B 25:1233–1236. doi:10.1107/S0567740869003876
Shein IR, Kiiko VS, Makurin YN, Gorbunova MA, Ivanovskii AL (2007) Elastic parameters of single-crystal and polycrystalline wurtzite-like oxides BeO and ZnO: Ab initio calculations. Phys Solid State 49:1067–1073
Kohout M (2011) DGRID, version 4.6. Radebeul
Sawada H, Wang R, Sleight AW (1996) An electron density residual study of zinc oxide. J Solid State Chem 122:148–150
Woll C (2007) The chemistry and physics of zinc oxide surfaces. Prog Surf Sci 82:55–120
Dulub O, Diebold U, Kresse G (2003) Novel stabilization mechanism on polar surfaces: ZnO(0001)-Zn. Phys Rev Lett 90:016102
Wander A, Schedin F, Steadman P, Norris A, McGrath R, Turner TS, Thornton G, Harrison NM (2001) Stability of polar oxide surfaces. Phys Rev Lett 86:3811–3814
Wander A, Harrison NM (2001) The stability of polar oxide surfaces: the interaction of H2O with ZnO(0001) and ZnO(000math). J Chem Phys 115:2312–2316. doi:10.1063/1.1384030
Meyer B (2004) First-principles study of the polar O-terminated ZnO surface in thermodynamic equilibrium with oxygen and hydrogen. Phys Rev B 69:045416
Jedrecy N, Sauvage-Simkin M, Pinchaux R (2000) The hexagonal polar ZnO(0001)-(1 x 1) surfaces: structural features as stemming from X-ray diffraction. Appl Surf Sci 162:69–73
King ST, Parihar SS, Pradhan K, Johnson-Steigelman HT, Lyman PF (2008) Observation of a (root 3 x root 3)R30 degrees reconstruction on O-polar ZnO surfaces. Surf Sci 602:L131–L134
Valtiner M, Torrelles X, Pareek A, Borodin S, Gies H, Grundmeier G (2010) In situ study of the polar ZnO(0001)-Zn surface in alkaline electrolytes. J Phys Chem C 114:15440–15447
Carlsson JM (2001) Electronic structure of the polar ZnO{0001}-surfaces. Comput Mater Sci 22:24–31
Humphrey W, Dalke A, Schulten K (1996) VMD—visual molecular dynamics. J Mol Graph 14:33–38
Xu PS, Sun YM, Shi CS, Xu FQ, Pan HB (2003) The electronic structure and spectral properties of ZnO and its defects. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms 199:286–290
Kaxiras E (2003) Atomic and electronic structure of solids. Cambridge University Press, Cambridge
Au CT, Hirsch W, Hirschwald W (1988) Adsorption of carbon-monoxide and carbon-dioxide on annealed and defect zinc-oxide (0001) surfaces studied by photoelectron-spectroscopy (Xps and Ups). Surf Sci 197:391–401
Chuasiripattana K, Warschkow O, Delley B, Stampfl C (2010) Reaction intermediates of methanol synthesis and the water-gas-shift reaction on the ZnO(0001) surface. Surf Sci 604:1742–1751
Lindsay R, Gutierrez-Sosa A, Thornton G, Ludviksson A, Parker S, Campbell CT (1999) NEXAFS study of CO adsorption on ZnO(000(1)over-bar1)-O and ZnO (000(1)over-bar)-O/Cu. Surf Sci 439:131–138
Kossmann J, Rossmuller G, Hattig C (2012) Prediction of vibrational frequencies of possible intermediates and side products of the methanol synthesis on ZnO(000(1)over-bar) by ab initio calculations. J Chem Phys 136:034706. doi:10.1063/1.3671450
Bowker M, Houghton H, Waugh KC (1981) Mechanism and kinetics of methanol synthesis on zinc oxide. J Chem Soc, Faraday Trans 1(77):3023–3036. doi:10.1039/F19817703023
Acknowledgments
The authors acknowledge the careful reading done by the referees. The authors are indebted to the financial support of National Council of Technological and Scientific Development (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and National Institute of Science and Technology of Materials in Nanotechnology (INCTMN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farias, S.A.S., Longo, E., Gargano, R. et al. CO2 adsorption on polar surfaces of ZnO. J Mol Model 19, 2069–2078 (2013). https://doi.org/10.1007/s00894-012-1636-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1636-4