Abstract
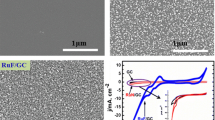
A simple procedure was developed to prepare a glassy carbon electrode modified with carbon nanotubes and Ruthenium (III) complexes. First, 25 μl of dimethyl sulfoxide–carbon nanotubes solutions (0.4 mg/ml) was cast on the surface of the glassy carbon electrode and dried in air to form a carbon nanotube film at the electrode surface. Then, the glassy carbon/carbon nanotube-modified electrode was immersed into a Ruthenium (III) complex solution (direct deposition) for a short period of time (10–20 s for multiwalled carbon nanotubes and 20–40 s for single-walled carbon nanotubes). The cyclic voltammograms of the modified electrode in aqueous solution shows a pair of well-defined, stable, and nearly reversible redox couple, Ru(III)/Ru(II), with surface-confined characteristics. The attractive mechanical and electrical characteristics of carbon nanostructures and unique properties and reactivity of Ru complexes are combined. The transfer coefficient (α), heterogeneous electron transfer rate constants (k s), and surface concentrations (Γ) for the glassy carbon/single-walled carbon nanotubes/Ru(III) complex-, glassy carbon/multiwalled carbon nanotubes/Ru(III) complex-, and glassy carbon/Ru(III) complex-modified electrodes were calculated using the cyclic voltammetry technique. The modified electrodes showed excellent catalytic activity, fast response time, and high sensitivity toward the reduction of nicotinamide adenine dinucleotide in phosphate buffer solutions at a pH range of 4–8. The catalytic cathodic current depends on the nicotinamide adenine dinucleotide concentration. In the presence of alcohol dehydrogenase, the modified electrode exhibited a response to addition of acetaldehyde. Therefore, the main product of nicotinamide adenine dinucleotide electroreduction at the Ru(III) complex/carbon nanotube-modified electrode was the enzymatically active NADH. The purposed sensor can be used for acetaldehyde determination.











Similar content being viewed by others
References
Aizawa M, Suzuki S, Kubo M (1976) Biochim Biophys Acta 444:886
Moiroux J, Deycard S, Malinski T (1985) J Electroanal Chem 194:99
Studnickova M, Klukanova HP, Turanek J, Kovar J (1988) J Electroanal Chem 252:383
Karyakin AA, Bobrova OA, Karyakina EE (1995) J Electroanal Chem 399:179
Damin A, Omanovic S (2006) J Mol Cat A Chem 253:222
Chen SM, Lin KH (2006) J Electroanal Chem 586:145
Chen SM, Lin KH (2005) J Electroanal Chem 583:248
Lin KC, Chen SM (2005) J Electroanal Chem 578:213
Karyakin AA, Bobrova OA, Karyakina EE (1995) J Electroanal Chem 399:179
Karyakin AA, Ivanova YN, Karyakina EE (2003) Electrochem Commun 5:677
Warriner K, Higson S Vadgama P (1997) Mater Sci Eng C 5:91
Beley M, Collin JP (1993) J Mol Catal 79:133
Man F, Omanovic S (2004) J Electroanal Chem 568:301
Damian A, Omanovic S (2006) J Mol Cat A: Chem 253:222
Lin KC, Chen SM (2006) J Electroanal Chem 589:52
Chen SM, Lin KH (2006) Electrochim Acta 51:4744
Sobolov SB, Leonida MD, Bartoszko-Malik A, Voivodov KI, McKinney F, Kim J, Fry AJ (1996) J Org Chem 61:2125
Voivodov KI, Sobolov SB, Leonida MD, Fry AJ (1995) Bioorg Med Chem Lett 5:681
Kim S, Yun SE, Kang C (1999) J Electroanal Chem 465:153
Kim S, Yun SE, Kang C (1999) Electrochem Commun 1:151
Cotton FA, Wilkinson G (1999) Advanced inorganic chemistry. Wiley, New York, pp 868–900
Appelbaum L, Heinriches C, Demtschuk J, Michman M, Oron M, Schafer HJ, Schumann H (1999) J Organomet Chem 592:240
Trasatti S (2000) Electrochim Acta 45:2377
Kim IH, Kim KB (2004) J Electrochem Soc 151:E7
Lima EC, Fenga PG, Romero JR, De- Giovani WF (1998) Polyhedron 17:313
Rodriguez M, Romero I, Liobet A, Deronzier AS, Parella T, Stoecki-Evans H (2001) Inorg Chem 40:4150
Premkumar J, Khoo SB (2004) Electrochem Commun 6:984
Wang X, Zhang Q, Han Z, Wang E, Guo Y, Hu C (2004) J Electroanal Chem 563:221
Azem A, Man F, Omanovic S (2004) J Molcul Cat A Chem 219:283
Salimi A, Pourbeyram S (2003) Talanta 60:205
Yan YK, Melchart M, Habtemariam A (2006) J Biol Inorg Chem 11:483
Polyanski D, Cabelli D, Muckerman JT, Fujita E, Koizumi TA, Fukushima T, Wada T, Tanaka K (2007) Angew Chem Int Ed 46:4169
Yakabson BI, Smally RE (1997) Am Sci 85:324
Lawrence NS, Wang J (2005) Electrochem Commun 8:71
Sun D, Zhu L, Huang H, Zhu G (2006) J Electroanal Chem 597:39
Zhao K, Song H, Zhung S, Dai L, He P, Fang Y (2007) Electrochem Commun 9:65
Li Z, Chen J, Pan D, Tao W, Nie L, Yao S (2006) Electrochim Acta 51:4255
Czerw R, Guo Z, Ajayan PM, Sun YP, Carol DL (2001) Nano Lett 1:423
Kooi SE, Schlecht U, Burghard M, Kern K (2002) Angew Chem 114:1409
Chen J, Liu H, Weimer WA, Halls MD, Waldeck DH, Walker GC (2002) J Am Chem Soc 124:9034
Chen RJ, Zhang Y, Wang D, Dai H (2001) J Am Chem Soc 123:3838
Frehill F, Vos JG, Benrezzak S, Koos AA, Konya Z, Ruther MG, Blau WJ, Fonseca A, Nagy JB, Biro LP, Minett AI, Panhuis M (2002) J Am Chem Soc 124:13694
Wang J (2005) Electroanalysis 17:7
Sherigara BS, Kutner W, Souza FD (2003) Electroanalysis 15:753
Davis JJ, Green MLH, Hill HAO, Leung YC, Sadler JO, Sloan JSC, Tsang SC (1998) Inorg Chim Acta 272:261
Tsang SC, Davis JJ, Green MLH, Hill HAO, Leung YC, Sadler JP (1995) J Chem Soc Chem Commun 1803
Davis JJ, Coles RJ, Hill HAO (1997) J Electroanal Chem 440:279
Wang J, Chen G, Wang M, Chatrathi MP (2004) Analyst 129:512
Hrapovic S, Liu YL, Male KB, Luong JHT (2004) Anal Chem 76:1083
Salimi A, Noorbakhsh A, Ghadermarzi M (2007) Sens Actuators B 123:530
Salimi A, Hallaj R (2005) Talanta 66:967
Salimi A, Noorbakhsh A, Ghadermarzi M (2005) Anal Biochem 344:16
Salimi A, Noorbakhsh A, Soltanian S (2006) Electroanalysis 18:16
Salimi A, Mamkhezri H, Mohebbi S (2006) Electrochem Commun 8:688
Sullivan BP, Calvert JM, Meyer TJ (1980) Inorg Chem 19:1404
Crutchley RJ, McCaw K, Lee FL, Gabe EJ (1990) Inorg Chem 29:2576
Bodige S, Mac Donnell FM (1997) Tetrahedron Lett 38:8159
Bard AJ, Faulkner LR (2001) Electrochemical methods, fundamentals and applications. Wiley, New York, p 231
Li J, Cassell A, Delzeit L, Han J, Meyyapan M (2002) J Phys Chem B 106:9299
Peigney A, Laurent C, Flahaut E, Bacsa RR, Rousset A (2001) Carbon 47:507
Laviron E (1974) J Electroanal Chem 52:355
Wang J (1994) Analytical electrochemistry. VCH, New York
Andriex CP, Saveant JM (1978) J Electroanal Chem 93:163
Acknowledgments
The financial supports of Iranian Nanotechnology inventive and Research Office of University of Kurdistan are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salimi, A., Izadi, M., Hallaj, R. et al. Electrocatalytic reduction of NAD+ at glassy carbon electrode modified with single-walled carbon nanotubes and Ru(III) complexes. J Solid State Electrochem 13, 485–496 (2009). https://doi.org/10.1007/s10008-008-0583-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-008-0583-6