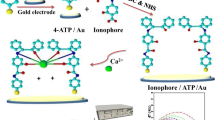
Abstract
Solid-state potentiometric calcium sensors based on newly synthesized Schiff’s base of 3-aminosalycilic acid with benzil [2-hydroxy-3-(2-oxo-1,2-diphenylethylidene)amino) benzoic acid] ionophore I and with isatin [2-hydroxy-3-(2-oxoindolin-3-ylidene amino)benzoic acid] ionophore II ionophores and their covalently attached to polyacrylamide ionophores III and IV, respectively, were developed. The all-solid-state sensors were constructed by the application of a thin film of polymeric membrane cocktail onto gold electrodes that were pre-coated with the conducting polymer poly (3,4-ethylenedioxy-thiophen) as an ion and electron transducer. More than 40 sensors with membranes containing plasticized PVC or poly(butyl methacrylate-co-dodecyl methacrylate as a plasticizer-free membrane matrix were investigated. The constructed sensors contained various amounts of the different ionophores with and without anionic lipophilic additive. The sensor containing 10% of ionophore III and 3% tetra (p-chlorophenyl) borate in acrylate copolymer exhibited a stable potentiometric response over a wide pH range of 4–9. It possessed a linear concentration range of 6 10−10 to 1 10−2 mol L−1 with a Nernstian slope of 28.5 mV/decade and a limit of detection (LOD) of 2 10−10 mol L−1. It exhibited a good selectivity for calcium to other cations. The selectivity coefficients towards different mono-, di- and trivalent cations were determined with the fixed interference method (FIM) and separate solution method (SSM). The sensor’s life time is more than 3 months, without significant deterioration in the slope. The proposed sensors were utilized for the determination of calcium concentration in serum. The results were compared with those obtained from routine clinical laboratory electrolyte analyser. The results reveal that the all-solid-state calcium sensor is promising for the point of care testing.










Similar content being viewed by others
References
Bogdanova A, Makhro A, Wang J, Lipp P, Kaestner L (2013) Calcium in red blood cells—a perilous balance. Intl J Mol Sci 14(5):9848
Bogdanova A, Makhro A, Wang J, Lipp P, Kaestner L. (2013), Calcium in red blood cells—a perilous balance, Int J Mol Sci 8;14(5):9848-72
Byrnes MC, Huynh K, Helmer SD, Stevens C, Dort JM, Smith RS (2005) A comparison of corrected serum calcium levels to ionized calcium levels among critically ill surgical patients
Kochegarov AA (2003) Pharmacological modulators of voltage-gated calcium channels and their therapeutical application. Cell Calcium 33(3):145–162
Young CC (1997) Evolution of blood chemistry analyzers based on ion selective electrodes. J Chem Edu 74(2):177
Petrukhin OM, ABK, Frakiisky EV, Urusov YI, Zhukov AF, Shipway AN, Baulin VE (2001) The effect of lipophilic anionic additives on detection limits of ion-selective electrodes based on ionophores with phosphoryl complexing groups. Sens Actuat B: Chemical 76(1–3):653–659
Ross JW (1967) Calcium-selective electrode with liquid ion exchanger. Science 156(3780):1378–1379
Bedlechowicz I, Sokalski T, Lewenstam A, Maj-Zurawska M (2005) Calcium ion-selective electrodes under galvanostatic current control. Sens Actuat B 108:836–839
Dimeski G, Badrick T, John AS (2010) Ion selective electrodes (ISEs) and interferences—a review. Clin Chim Acta 411(5–6):309–317
Abbas MN, Radwan AA (2008) Novel lipoate-selective membrane sensor for the flow injection determination of α-lipoic acid in pharmaceutical preparations and urine. Talanta 74:1113–1121
Abbas MN, Amer HS (2013) Solid contact indium (III) sensor based on Thiosulfinate Ionophore derived from omeprazole. Bull Kor Chem Soc 34:1153–1159
Bobacka J, Ivaska A, Lewenstam A (2003) Potentiometric ion sensors based on conducting polymers. Electroanalysis 15(5–6):366–374
Zine N, Bausells J, Ivorra A, Aguiló J, Zabala M, Teixidor F, Masalles C, Viñas C, Errachid A (2003) Hydrogen-selective microelectrodes based on silicon needles. Sens Actuat B: Chemical 91(1–3):76–82
Radu A, Peper S, Gonczy C, Runde W, Diamond D (2006) Trace-level determination of Cs+ using membrane-based ion-selective electrodes. Electroanalysis 18(13–14):1379–1388
Vázquez M, Danielsson P, Bobacka J, Lewenstam A, Ivaska A (2004) Solution-cast films of poly(3,4-ethylenedioxythiophene) as ion-to-electron transducers in all-solid-state ion-selective electrodes. Sens Actuat B: Chemical 97(2–3):182–189
Abbas MN, Radwan AA, Bühlmann P, Abd El Ghaffar MA (2011) Solid-contact perchlorate sensor with Nanomolar detection limit based on cobalt phthalocyanine ionophores covalently attached to polyacrylamide. AJAC 2:820–831
Pandey PC, Prakash R (1998) Polyindole modified potassium ion-sensor using dibenzo-18-crown-6 mediated PVC matrix membrane. Sens Actuat B: Chemical 46(1):61–65
Correia ADP, Magalhães SJMC, Machado CAAS (2008) Array of potentiometric sensors for multicomponent analysis of blood serum. Microchim Acta 163(1):131–137
Wang S-H, Chou T-C, Liu C-C (2003) Development of a solid-state thick film calcium ion-selective electrode. Sens Actuat B: Chemical 96(3):709–716
Michalska A, Konopka A, Maj-Zurawska M (2003) All-solid-state calcium solvent polymeric membrane electrode for low-level concentration measurements. Anal Chem 75(1):141–144
I. Bedlechowicz TS, A. Lewenstam, M. Maj-Zurawska (2005) Calcium ion-selective electrodes under galvanostatic current control. Sens Actuat. B 108 836–839
Kumar A, Mittal SK (2004) PVC based dibenzo-18-crown-6 electrode for Ca(II) ions. Sens Actuat B: Chemical 99(2–3):340–343
Balaban T S, Constantieux T, Cahard D, Campagne J, Chataigner I, (2014) Science of synthesis: Houben-Weyl methods of molecular transformations Vol. 26: Ketones, Georg Thieme Verlag, p 3873
Peper S, Ceresa A, Qin Y, Bakker E (2003) Plasticizer-free microspheres for ionophore-based sensing and extraction based on a methyl methacrylate-decyl methacrylate copolymer matrix. Anal Chim Acta 500(1–2):127–136
The chemistry of heterocyclic compounds, indoles part one edited by William J. Houlihan, John Wiley & Sons, Sep 15, 2009- Science - page 184
Liua Y, ue Y, Tang H, Wang M, Qin Y (2012) Click-immobilized K+-selective ionophore for potentiometric and optical sensors. Sens. Actuat 171(1):556–562
Daunert S, Bachas LG (1990) Ion-selective electrodes using an ionophore covalently attached to carboxylated poly(vinyl chloride). Anal Chem 62:1428–1431
Ganjali MR, Zamani HA, Norouzi P, Adib M, Accedyc M (2005) Novel calcium sensor based on [2-(2-hydroxyphenyl)imino]-1,2-diphenylethanone. Acta Chim Slov 52:309–316
Bakker E, Pretsch E (2005) Potentiometric sensors for trace-level analysis. Trends Anal Chem 24:199
Veder J, De Marco R, Clarke G, Chester R, Nelson A, Prince K, Pretsch E, Bakker E (2008) Elimination of undesirable water layers in solid-contact polymeric ion-selective electrodes. Anal Chem 80:6731
Abbas MN, Radwan AA, Nawwar GA, Zine N, Errachid A (2015) A durable solid contact sulfide sensor based on a ceric acrylohydrazide ionophore attached to polyacrylamide with a nanomolar detection limit. Anal Methods 7:7930–7942
Zine N, Bausells J, Vocanson F, Lamartine R, Asfari Z, Teixidor F, Crespo E, de Oliveira IAM, Samitier J, Errachid A (2006) Potassium-ion selective solid contact microelectrode based on a novel 1,3-(di-4-oxabutanol)-calix[4]arene-crown-5 neutral carrier. Electrochim Acta 51(24):5075–5079
Hu J, Stein A, Buhlmann P (2016) Rational design of all-solid-state ion-selective electrodes and reference electrodes. Trends Anal Chem 76:102–114
Fukagawa M, Kurokawa K, Papadakis MA (2007) Current medical diagnosis and treatment. In: McPhee SJ, Papadakis MA, Tierney LM Jr (eds) Fluid & electrolyte disorders. McGraw Hill, New York, NY, p 2007
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abbas, M.N., Magar, H.S. Highly sensitive and selective solid-contact calcium sensor based on Schiff base of benzil with 3-aminosalycilic acid covalently attached to polyacrylic acid amide for health care. J Solid State Electrochem 22, 181–192 (2018). https://doi.org/10.1007/s10008-017-3727-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3727-8