Abstract
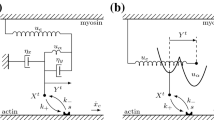
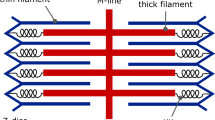
The HMK model (Hunter et al. in Prog Biophys Mol Biol 69:289–331, 1998) proposes mechanobiological equations for the influence of intracellular calcium concentration \(\hbox {Ca}_\mathrm{i}\) on the evolution of bound calcium concentration \(\hbox {Ca}_\mathrm{b}\) and the tropomyosin kinetics parameter z, which model processes in the active component of the tension in cardiac muscle. The inelastic response due to actin-myosin crossbridge kinetics is modeled in the HMK model with a function Q that depends on the history of the rate of total stretch of the muscle fiber. Here, an alternative model is proposed which models the active component of the muscle fiber as a viscoplastic material. In particular, an evolution equation is proposed for the elastic stretch \(\lambda _\mathrm{a} \) in the active component. Specific forms of the constitutive equations are proposed and used to match experimental data. The proposed viscoplastic formulation allows for separate modeling of three processes: the high rate deactivation of crossbridges causing rapid reduction in active tension; the high but lower rate reactivation of crossbridges causing recovery of active tension; and the low rate relaxation effects characterizing the Hill model of muscles.









Similar content being viewed by others
References
Ambrosi D, Pezzuto S (2012) Active stress vs. active strain in mechanobiology: constitutive issues. J Elast 107:199–212
Berman MR, Peterson JN, Yue DT, Hunter WC (1988) Effect of isoproterenol on force transient time course and on stiffness spectra in rabbit papillary muscle in barium contracture. J Mol Cell Cardiol 20:415–426
Campbell KB, Chandra M, Kirkpatrick RD, Slinker BK, Hunter WC (2004) Interpreting cardiac muscle force-length dynamics using a novel functional model. Am J Physiol Heart Circ Physiol 286:H1445–H1525
Campbell KB, Taheri H, Kirkpatrick RD, Burton T, Hunter WC (1993) Similarities between dynamic elastance of left ventricular chamber and papillary muscle of rabbit heart. Am J Physiol Heart Circ Physiol 264:H1926–H1941
Chabiniok R, Moireau P, Lesault PF, Rahmouni A, Deux JF, Chapelle D (2012) Estimation of tissue contractility from cardiac cine-MRI using a biomechanical heart model. Biomech Model Mechanobiol 11:609–630
Costa KD, Holmes JW, McCulloch AD (2001) Modelling cardiac mechanical properties in three dimensions. Philos Trans R Soc Lond A359:1233–1250
Ford LE, Huxley AF, Simmons RM (1977) Tension responses to sudden length change in stimulated frog muscle fibers near slack length. J Physiol 269:441–515
Guccione JM, Waldman LK, McCulloch AD (1993) Mechanics of active contractions in cardiac muscle: Part II—cylindrical models of the systolic left ventricle. ASME J Biomech Eng 115:83–90
Hadjicharalambous M, Chabiniok R, Asner L, Smmut E, Wong J, Car-White G, Lee J, Razavi R, Smith N, Norsletten D (2015) Analysis of passive cardiac constitutive laws for parameter estimation using 3D tagged MRI. Biomech Model Mechanobiol 14:807–828
Hancock WO, Martyn DA, Huntsman LL (1993) \(Ca^{2+}\) and segment length dependence of isometric force kinetics in intact ferret cardiac muscle. Circ Res 73:603–611
Hill AV (1938) Heat of shortening and the dynamic constants of muscle. Proc R Soc Ser B 126:136–195
Holzapfel GA, Ogden RW (2009) Constitutive modeling of passive myocardium: a structurally based framework for material characterization. Philos Trans R Soc A367:3445–3475
Humphrey JD, Strumpf RK, Yin FCP (1990) Determination of a constitutive relation for passive myocardium: I a new functional form. J Biomech Eng 112:333–339
Hunter PJ, McCulloch AD, ter Keurs HEDJ (1998) Modelling the mechanical properties of cardiac muscle. Prog Biophys Mol Biol 69:289–331
Lee EH (1969) Elasto-plastic deformation at finite strain. J Appl Mech 36:1–6
Nardinocchi P, Teresi L (2007) On the active response of soft living tissues. J Elast 88:27–39
Nash MP, Hunter PJ (2000) Computational mechanics of the heart. From tissue structure to ventricular function. J Elast 61:113–141
Niederer SA, Hunter PJ, Smith NP (2006) A quantitative analysis of cardiac myocyte relaxation: a simulation study. Biophys J 90:1697–1722
Rice JJ, deTombe PP (2004) Approaches to modeling crossbridges and calcium-dependent activation in cardiac muscle. Prog Biophys Mol Biol 85:179–195
Rodriguez EK, Hoger A, McCulloch AD (1994) Stress-dependent finite growth in soft elastic tissues. J Biomech 27:455–467
Rossi S, Ruiz-Baier R, Pavarino LF, Quarteroni A (2012) Orthotropic active strain models for the numerical simulation of cardiac biomechanics. Int J Numer Method Biomed Eng 28:761–788
Rossmanith GH, Hoh JFY, Kirman A, Kwan LJ (1986) Influence of V1 and V3 isomyosins on the mechanical behaviour of rat papillary muscle as studied by pseud-random noise modulated length perturbations. J Muscle Res Cell Motil 7:307–319
Rubin MB, Safadi MM, Jabareen M (2015) A unified theoretical structure for modeling interstitial growth and muscle activation in soft tissues. Int J Eng Sci 90:1–26
Taber LA (1991) On a nonlinear theory for muscle shells: Part II—application to the beating left ventricle. J Biomech Eng 113:63–71
Taber LA, Perucchio R (2000) Modeling heart development. J Elast 61:165–197
Wang VY, Lam HI, Ennis DB, Cowan BR, Young AA, Nash MP (2009) Modelling passive diastolic mechanics with quantitative MRI of cardiac structure and function. Med Image Anal 13:773–784
Acknowledgments
This research was partially supported by MB Rubin’s Gerard Swope Chair in Mechanics. MB Rubin would also like to acknowledge helpful discussions with PJ Hunter and the generosity of the Auckland Bioengineering Institute for hosting him during part of his sabbatical leave from Technion.
Author information
Authors and Affiliations
Corresponding author
Appendix: Details of the analysis of the dynamic stiffness
Appendix: Details of the analysis of the dynamic stiffness
Substituting (88) into (6), (7) and (34) yields (89) where the functions \(f_{i}\) and \(C_{i}\) are given by
with R being set equal to unity in all of the equations in this appendix. Linearizing these functions about the homeostatic state (85) yields the following values for \(A_{ij}, B_{j}\) and \(C_{j}\) in (91)
Rights and permissions
About this article
Cite this article
Rubin, M.B. A viscoplastic model for the active component in cardiac muscle. Biomech Model Mechanobiol 15, 965–982 (2016). https://doi.org/10.1007/s10237-015-0736-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-015-0736-3