Abstract
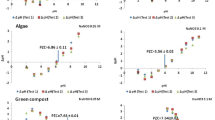
Potentiometric mass titration (PMT) technique has been adapted to determine the pH pzc of four vegetable wastes: grape stalks, cork, yohimbe bark and olive stones wastes used for Cu(II) removal. The pH at the point zero charge (pH pzc), determined by PMT, are compared with that obtained by two classical techniques: mass titration (MT) and immersion technique (IT). PMT has been found to be an easy and appropriate technique to determine pH pzc of the studied materials. From the results, the knowledge of sorbents pH pzc provides information about the possible attraction and repulsion between sorbent and sorbate but in any case enables to ensure that electrostatic force is one of the mechanisms that takes place in metal sorption.




Similar content being viewed by others
References
Bourikas K, Vakros J, Kordulis C, Lycourghiotis A (2003) Potentiometric mass titrations: experimental and theoretical establishment of a new technique for determining the point of zero charge (PZC) for metal (Hydr)oxides. J Phys Chem 107:9441–9451
Cochrane E, Lu S, Gibb SW, Villaescusa I (2006) A comparison of low-cost biosorbents and commercial sorbents for the removal of copper from aqueous media. J Hazard Mater B137:198–206 doi:10.1016/j.hazmat.2006.01.054
Dupont L, Bouanda J, Ghanbaja J, Dumonceau J, Aplincourt M (2004) Use of analytical microscopy to analyze the speciation of copper and chromium ions onto a low-cost biomaterial. J Colloid Interface Sci 279:418–424 doi:10.1016/j.jcis.2004.06.069
Escudero C, Fiol N, Poch J, Villaescusa I (2008) The kinetics of copper sorption onto yohimbe bark wastes. Int J Environ Pollut (in press)
Fiol N, Villescusa I, Martínez M, Miralles N, Poch J, Serarols J (2006) Sorption of Pb(II), Ni(II), Cu(II) and Cd(II) from aqueous solution by olive stone wastes. Sep Purif Technol 50:132–140 doi:10.1016/j.seppur.2005.11.016
Faur-Brasquet C, Reddad Z, Kadirvelu K, Le Clored P (2002) Modelling the adsorption of metal ions (Cu2+,Ni2+,Pb2+) onto ACCS using surface complexation models. Appl Surf Sci 196:356–365
Gérente C, Couespel du Mesnil P, Andrès Y, Thibault J, Le Cloirec P (2000) Removal of metal ions from aqueous solution on low cost natural polysaccharides. Sorption mechanism approach. React Funct Polym 46:135–144
Kikuchi Y, Qian Q, Machida M, Tatsumoto H (2006) Effect of ZnO loading to activated carbon on pB(II) adsorption from aqueous solution. Carbon 44:195–202 doi:10.1016/j.carbon.2005.07.040
Liu Y, Chang X, Guo Y, Meng S (2006) Biosorption and preconcentration of lead and cadmium on waste Chinese herb Pang Da Hai. J Hazard Mater B135:389–394 doi:10.1016/j.hazmat.2005.11.078
Montanher SF, Oliveira EA, Rollemberg MC (2005) Removal of metal ions from aqueous solutions by sorption onto rice bran. J Hazard Mater B117:207–211 doi:10.1016/j.jhazmat.2004.09.015
Nurchi VM, Floris C, Pinna R, Fiol N, Villaescusa I (2007) Metal ion uptake from aqueous solution by olive stones: a carbon-13 solid-state nuclear magnetic resonance and potentiometric study. Water Environ Res 79:2363–2367
Panda GC, Das SK, Chatterjee S, Maity PB, Bandopadhyay TS, Guha AK (2006) Adsorption of cadmium on husk of Lathyrus sativus: physico-chemical study. Colloids Surf B Biointerfaces 50(1):49–54 doi:10.1016/j.colsurfb.2006.03.022
Puigdomènech I (2004) Chemical Equilibrium software (1.6 Mb) http://web.telia.com/~u15651596/, updated on 18 February 2004, downloaded in May 2006
Saeed A, Akhter MW, Iqbal M (2005) Removal and recovery of heavy metals from aqueous solution using papaya wood as a new biosorbent. Sep Purif Technol 45:25–31 doi:10.1016/j.seppur.2005.02.004
Taty-Costodes VC, Fauduet H, Porte C, Delacroix A (2003) Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. J Hazard Mater B105:121–142 doi:10.1016/j.jhazmat.2003.07.009
Villaescusa I, Martínez M, Miralles N (2000) Heavy metal uptake from aqueous solution by cork and yohimbe bark wastes. J Chem Technol Biotechnol 75:812–816
Villaescusa I, Fiol N, Cristiani F, Floris C, Lai S, Nurchi VM (2002) Copper(II) and Nickel (II) uptake from aqueous solutions by cork wastes: a NMR and potentiometric study. Polyhedron 21:1363–1367
Villaescusa I, Fiol N, Martínez M, Miralles N, Poch J, Serarols J (2004) Removal of copper and nickel ions from aqueous solutions by grape stalks wastes. Water Res 38:992–1002 doi:10.1016/j.watres.2003.10.040
Acknowledgments
This research was supported by the Ministerio de Educación y Ciencia, Spain (project CTM2005-07342-C02-01/TECNO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fiol, N., Villaescusa, I. Determination of sorbent point zero charge: usefulness in sorption studies. Environ Chem Lett 7, 79–84 (2009). https://doi.org/10.1007/s10311-008-0139-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-008-0139-0