Abstract
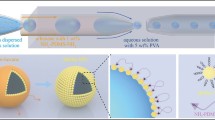
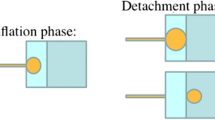
The heatable microfluidic chip developed herein successfully integrates a microheater and flow-focusing device to generate uniform-sized gelatin emulsions under various flow rate ratios (sample phase/oil phase, Q s/Q o) and driven voltages. The gelatin emulsions can be applied to encapsulate vitamin C for drug release. Our goal is to create the thermal conditions for thermo-sensitive hydrogel materials in the microfluidic chip and generate continuous and uniform emulsions under any external environment. The gelatin emulsion sizes have a coefficient of variation of <5 % and can be precisely controlled by altering the flow rate ratio (Q s/Q o) and driven voltage. The gelatin emulsion diameters range from 45 to 120 μm. Moreover, various sizes of these gelatin microcapsules containing vitamin C were used for drug release. The developed microfluidic chip has the advantages of a heatable platform in the fluid device, active control over the emulsion diameter, the generation of uniform-sized emulsions, and simplicity. This new approach for gelatin microcapsules will provide many potential applications in drug delivery and pharmaceuticals.








Similar content being viewed by others
References
Adamson DN, Mustafi D, Zhang JXJ, Zheng B, Ismagilov RF (2006) Production of arrays of chemically distinct nanolitre plugs via repeated splitting in microfluidic devices. Lab Chip 6:1178–1186
Anna SL, Bontoux N, Stone HA (2003) Formation of dispersions using flow focusing in microchannels. Appl Phys Lett 82:364–366
Asai A (1991) Bubble dynamics in boiling under high heat flux pulse heating. J Heat Transf 113:973–979
Bruschi ML, Cardoso MLC, Lucchesi MB, Gremião MPD (2003) Gelatin microparticles containing propolis obtained by spray-drying technique: preparation and characterization. Int J Pharm 264:45–55
Butler MF, Paul YF, Pudney DA (2003) Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J Polym Sci Part A Polym Chem 41:3941–3953
Choi CH, Jung JH, Rhee YW, Kim DP, Shim SE, Lee CS (2007) Generation of monodisperse alginate microbeads and in situ encapsulation of cell in microfluidic device. Biomed Microdevices 9:855–862
Djabourov M, Papon P (1983) Influence of thermal treatments on the structure and stability of gelatin gels. Polymer 24:524–537
Gombotz WR, Wee SF (1998) Protein release from alginate matrices. Adv Drug Deliv Rev 31:267–285
Hoesli CA, Raghuram K, Kiang RLJ, Mocinecova D, Hu X, Johnson JD, Lacik I, Kieffer TJ, Piret JM (2011) Pancreatic cell immobilization in alginate beads produced by emulsion and internal gelation. Biotechnol Bioeng 108:424–434
Huang KS, Lai TH, Lin YC (2006a) Manipulating the generation of Ca-alginate microspheres using microfluidic channels as a carrier of gold nanoparticles. Lab Chip 6:954–957
Huang KS, Lai TH, Lin YC (2006b) Using a microfluidic chip and internal gelation reaction for monodisperse calcium alginate microparticles generation. Front Biosci 12:3061–3067
Huang KS, Lu K, Yeh CS, Chung SR, Lin CH, Yang CH, Dong YS (2009) Microfluidic controlling monodisperse microdroplet for 5-fluorouracil loaded genipin-gelatin microcapsules. J Controlled Release 137:15–19
Hung LH, Teh SY, Jester J, Lee AP (2010) PLGA micro/nanosphere synthesis by droplet microfluidic solvent evaporation and extraction approaches. Lab Chip 10:1820–1825
Kawakatsu T, Tragardh G, Tragardh Ch, Nakajima M, Oda N, Yonemoto T (2001) The effect of the hydrophobicity of microchannels and components in water and oil phases on droplet formation in microchannel water-in-oil emulsification. Colloids Surf 179:29–37
Kikuchi A, Okano T (2002) Pulsatile drug release control using hydrogels. Adv Drug Deliv Rev 54:53–77
Kopp MU, de Mello AJ, Manz A (1998) Chemical amplification: continuous-flow PCR on a chip. Science 280:1046–1048
Kushibiki T, Tomoshige R, Fukunaka Y, Kakemi M, Tabata Y (2003) In vivo release and gene expression of plasmid DNA by hydrogels of gelatin with different cationization extents. J Controlled Release 90:207–216
Lagally ET, Emrich CA, Mathies RA (2001) Fully integrated PCR-capillary electrophoresis microsystem for DNA analysis. Lab Chip 1:102–107
Lan WJ, Li SW, Xu JH, Luo GS (2010) One-step synthesis of chitosan–silica hybrid microspheres in a microfluidic device. Biomed Microdevices 12:1087–1095
Li W, Nie Z, Zhang H, Paquet C, Seo M, Garstecki P, Kumacheva E (2007) Screening of the effect of surface energy of microchannels on microfluidic emulsification. Langmuir 23:8010–8014
Li W, Young EWK, Seo M, Nie Z, Garstecki P, Simmons CA, Kumacheva E (2008) Simultaneous generation of droplets with different dimensions in parallel integrated microfluidic droplet generators. Soft Matter 4:258–262
Link DR, Mongrain EG, Duri A, Sarrazin F, Cheng Z, Cristobal G, Marquez M, Weitz DA (2006) Electric control of droplets in microfluidic devices. Angew Chem 118:2618–2622
Liu K, Ding HJ, Liu J, Chen Y, Zhao XZ (2006) Shape-controlled production of biodegradable calcium alginate gel microparticles using a novel microfluidic device. Langmuir 22:9453–9457
Murshed SMS, Tan SH, Nguyen NT, Wong TN, Yobas L (2009) Microdroplet formation of water and nanofluids in heat-induced microfluidic T junction. Microfluid Nanofluid 6:253–259
Nisisako T, Torii T, Takahashi T, Takizawa Y (2006) Synthesis of monodisperse bicolored Janus particles with electrical anisotropy using a microfluidic co-flow system. Adv Mater 18:1152–1156
Owen MJ, Smith PJ (1994) Plasma treatment of polydimethysiloxane. J Adhes Sci Technol 8:1063–1075
Rao VS, Kripesh V, Yoon SW, Tay AAO (2006) A thick photoresist process for advanced wafer level packaging applications using JSR THB-151N negative tone UV photoresist. J Micromech Microeng 16:1841–1846
Roy S, Pal M, Gupta BK (1992) Indomethacin-loaded microspheres: design and preparation by a multiple-emulsification technique and their in vitro evaluation. Pharm Res 9:1132–1136
Serra C, Berton N, Bouquey M, Prat L, Hadziioannou G (2007) A predictive approach of the influence of the operating parameters on the size of polymer particles synthesized in a simplified microfluidic system. Langmuir 23:7745–7750
Sivakumar M, Rao KP (2003) Preparation, characterization, and in vitro release of gentamicin from coralline hydroxyapatite–alginate composite microspheres. Biomaterials 23:3175–3181
Srivastava AK, Ridhurkar DN, Wadhwa S (2005) Floating microspheres of cimetidine: formulation, characterization and in vitro evaluation. Acta Pharm 55:277–285
Vandervoort J, Ludwig A (2004) Preparation and evaluation of drug-loaded gelatin nanoparticles for topical ophthalmic use. Eur J Pharm Biopharm 57:251–261
Wang LY, Ma GH, Su ZG (2005) Preparation of uniform sized chitosan microspheres by membrane emulsification technique and application as a carrier of protein drug. J Controlled Release 106:62–75
Xu Q, Hashimoto M, Dang TT, Hoare T, Kohane DS, Whitesides GM, Langer R, Andersonl DG (2009) Preparation of monodisperse biodegradable polymer microparticles using a microfluidic flow-focusing device for controlled drug delivery. Small 5:1575–1581
Yang LJ, Wang JM, Huang YL (2004) The micro ion drag pump using indium-tin-oxide (ITO) electrodes to resist aging. Sens Actuators A Phys 111:118–122
Yang CH, Huang KS, Lin PW, Lin YC (2007) Using a cross-flow microfluidic chip and external crosslinking reaction for monodisperse TPP-chitosan microparticles. Sens Actuators B Chem 124:510–516
Yeh CH, Lin YC (2009) Using a cross-flow microfluidic chip for monodisperse UV-photopolymerized microparticles. Microfluid Nanofluid 6:277–283
Yeh CH, Zhao Q, Lee SJ, Lin YC (2009) Using a T junction microfluidic chip for monodisperse calcium alginate microparticles and encapsulation of nanoparticles. Sens Actuators A Phys 151:231–236
Yeh CH, Lin PW, Lin YC (2010) Chitosan microfiber fabrication using a microfluidic chip and its application to cell cultures. Microfluid Nanofluid 8:115–121
Yeh CH, Chen YC, Lin YC (2011) Generation of droplets with different concentrations by using gradient-microfluidic droplet generator. Microfluid Nanofluid 11:245–253
Yeh CH, Lee MH, Lin YC (2012) Using an electro-spraying microfluidic chip to produce uniform emulsions under a direct-current electric field. Microfluid Nanofluid 12:475–484
Young S, Wong M, Tabata Y, Mikos G (2005) Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Controlled Release 109:256–274
Zheng B, Roach LS, Ismagilov RF (2003) Screening of protein crystallization conditions on a microfluidic chip using nanoliter-size droplets. J Am Chem Soc 125:11170–11171
Acknowledgments
The authors would like to thank the Center for Micro/Nano Science and Technology, National Cheng Kung University, Tainan, Taiwan, ROC, for access to equipment and technical support. Funding from the National Science Council of Taiwan, ROC, under grants NSC 101-2811-E-006-051 and 101-2221-E-006-192-MY2 is gratefully acknowledged. The work was also partially supported by the “Aim for the Top University Plan” of the National Chiao Tung University and Ministry of Education, Taiwan, ROC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeh, CH., Chen, KR. & Lin, YC. Developing heatable microfluidic chip to generate gelatin emulsions and microcapsules. Microfluid Nanofluid 15, 775–784 (2013). https://doi.org/10.1007/s10404-013-1193-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-013-1193-x