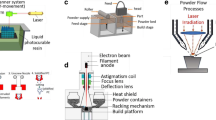
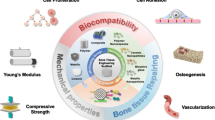
Abstract
The need for bone grafts is tremendous, and that leads to the use of autograft, allograft, and bone graft substitutes. The biology of the bone is quite complex regarding cellular composition and architecture, hence developing a mineralized connective tissue graft is challenging. Traditionally used bone graft substitutes including metals, biomaterial coated metals and biodegradable scaffolds, suffer from persistent limitations. With the advent and rise of additive manufacturing technologies, the future of repairing bone trauma and defects seems to be optimistic. 3D printing has significant advantages, the foremost of all being faster manipulation of various biocompatible materials and live cells or tissues into the complex natural geometries necessary to mimic and stimulate cellular bone growth. The advent of new-generation bioprinters working with high-precision, micro-dispensing and direct digital manufacturing is aiding in ground-breaking organ and tissue printing, including the bone. The future bone replacement for patients holds excellent promise as scientists are moving closer to the generation of better 3D printed bio-bone grafts that will be safer and more effective. This review aims to summarize the advances in scaffold fabrication techniques, emphasizing 3D printing of biomimetic bone grafts.






Similar content being viewed by others
References
Abbah, S. A., J. Liu, R. W. M. Lam, J. C. H. Goh, and H. K. Wong. In vivo bioactivity of rhBMP-2 delivered with novel polyelectrolyte complexation shells assembled on an alginate microbead core template. J. Control. Release 162:364–372, 2012.
Aboudzadeh, N., M. Imani, M. A. Shokrgozar, A. Khavandi, J. Javadpour, Y. Shafieyan, and M. Farokhi. Fabrication and characterization of poly(d, l-lactide-co-glycolide)/hydroxyapatite nanocomposite scaffolds for bone tissue regeneration. J. Biomed. Mater. Res. Part A 94:137–145, 2010.
Agarwal, R., and A. J. Garcia. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 94:53–62, 2015.
Alaribe, F. N., S. L. Manoto, and S. C. K. M. Motaung. Scaffolds from biomaterials: Advantages and limitations in bone and tissue engineering. Biologia 71:353–366, 2016.
Alizadeh-Osgouei, M., Y. Li, and C. Wen. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioactive Mater. 4:22–36, 2019.
Alvarez, K., and H. Nakajima. Metallic Scaffolds for bone regeneration. Materials 2:790–832, 2009.
Amosi, N., S. Zarzhitsky, E. Gilsohn, O. Salnikov, E. Monsonego-Ornan, R. Shahar, and H. Rapaport. Acidic peptide hydrogel scaffolds enhance calcium phosphate mineral turnover into bone tissue. Acta Biomater. 8:2466–2475, 2012.
Aranaz, I., M. Mengibar, R. Harris, I. Panos, B. Miralles, N. Acosta, G. Galed, and A. Heras. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 3:203–230, 2009.
Aszodi, A., J. F. Bateman, E. Gustafsson, R. Boot-Handford, and R. Fassler. Mammalian skeletogenesis and extracellular matrix: What can we learn from knockout mice? Cell Struct. Funct. 25:73–84, 2000.
Baldwin, P., D. J. Li, D. A. Auston, H. S. Mir, R. S. Yoon, and K. J. Koval. Autograft, allograft, and bone graft substitutes. J. Orthop. Trauma 33:203–213, 2019.
Barron, J. A., P. Wu, H. D. Ladouceur, and B. R. Ringeisen. Biological laser printing: A novel technique for creating heterogeneous 3-dimensional cell patterns. Biomed. Microdev. 6:139–147, 2004.
Beederman, M., J. D. Lamplot, G. Nan, J. Wang, X. Liu, L. Yin, R. Li, W. Shui, H. Zhang, S. H. Kim, W. Zhang, J. Zhang, Y. Kong, S. Denduluri, M. R. Rogers, A. Pratt, R. C. Haydon, H. H. Luu, J. Angeles, L. L. Shi, and T. C. He. BMP signaling in mesenchymal stem cell differentiation and bone formation. J. Biomed. Sci. Eng. 6:32–52, 2013.
Beuttel, E., N. Bormann, A.-M. Pobloth, G. Duda, and B. Wildemann. Impact of gentamicin-loaded bone graft on defect healing in a sheep model. Materials 12:1116, 2019.
Bhattacharjee, P., B. Kundu, D. Naskar, H. W. Kim, T. K. Maiti, D. Bhattacharya, and S. C. Kundu. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 63:1–17, 2017.
Bigham-Sadegh, A., and A. Oryan. Selection of animal models for pre-clinical strategies in evaluating the fracture healing, bone graft substitutes and bone tissue regeneration and engineering. Connect. Tissue Res. 56:175–194, 2015.
Bishop, E. S., S. Mostafa, M. Pakvasa, H. H. Luu, M. J. Lee, J. M. Wolf, G. A. Ameer, T. C. He, and R. R. Reid. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 4:185–195, 2017.
Bose, S., M. Roy, and A. Bandyopadhyay. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 30:546–554, 2012.
Bose, S., S. Vahabzadeh, and A. Bandyopadhyay. Bone tissue engineering using 3D printing. Mater. Today 16:496–504, 2013.
Bouet, G., D. Marchat, M. Cruel, L. Malaval, and L. Vico. In vitro three-dimensional bone tissue models: from cells to controlled and dynamic environment. Tissue Eng. Part B 21:133–156, 2015.
Brunello, G., S. Panda, L. Schiavon, S. Sivolella, L. Biasetto, and M. Del Fabbro. The impact of bioceramic scaffolds on bone regeneration in preclinical in vivo studies: A systematic review. Materials 13:1500, 2020.
Campbell, P. G., and L. E. Weiss. Tissue engineering with the aid of inkjet printers. Exp. Opin. Biol. Ther. 7:1123–1127, 2007.
Capulli, M., R. Paone, and N. Rucci. Osteoblast and osteocyte: Games without frontiers. Arch. Biochem. Biophys. 561:3–12, 2014.
Charles, J. F., and A. O. Aliprantis. Osteoclasts: More than ‘bone eaters’. Trends Mol. Med. 20:449–459, 2014.
Chen, C. Y., C. J. Ke, K. C. Yen, H. C. Hsieh, J. S. Sun, and F. H. Lin. 3D porous calcium-alginate scaffolds cell culture system improved human osteoblast cell clusters for cell therapy. Theranostics 5:643–655, 2015.
Chen, Q. Z., Y. Li, L. Y. Jin, J. M. Quinn, and P. A. Komesaroff. A new sol-gel process for producing Na(2)O-containing bioactive glass ceramics. Acta Biomater. 6:4143–4153, 2010.
Chen, Q. Z., K. Rezwan, V. Francon, D. Armitage, S. N. Nazhat, F. H. Jones, and A. R. Boccaccini. Surface functionalization of Bioglass-derived porous scaffolds. Acta Biomater. 3:551–562, 2007.
Chen, J., Z.-D. Shi, X. Ji, J. Morales, J. Zhang, N. Kaur, and S. Wang. Enhanced osteogenesis of human mesenchymal stem cells by periodic heat shock in self-assembling peptide hydrogel. Tissue Eng. Part A 19:716–728, 2013.
Chen, Q. Z., I. D. Thompson, and A. R. Boccaccini. 45S5 Bioglass-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials 27:2414–2425, 2006.
Cheong, V. S., P. Fromme, M. J. Coathup, A. Mumith, and G. W. Blunn. Partial bone formation in additive manufactured porous implants reduces predicted stress and danger of fatigue failure. Ann. Biomed. Eng. 48:502–514, 2020.
Chia, H. N., and B. M. Wu. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 9:1–14, 2015.
Chocholata, P., V. Kulda, and V. Babuska. Fabrication of Scaffolds for bone-tissue regeneration. Materials 12:568, 2019.
Chung, C., and J. A. Burdick. Engineering cartilage tissue. Adv. Drug Deliv. Rev. 60:243–262, 2008.
Cipitria, A., J. C. Reichert, D. R. Epari, S. Saifzadeh, A. Berner, H. Schell, M. Mehta, M. A. Schuetz, G. N. Duda, and D. W. Hutmacher. Polycaprolactone scaffold and reduced rhBMP-7 dose for the regeneration of critical-sized defects in sheep tibiae. Biomaterials 34:9960–9968, 2013.
Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 3:S131–S139, 2008.
Collins, M. N., and C. Birkinshaw. Hyaluronic acid based scaffolds for tissue engineering-A review. Carbohydr. Polym. 92:1262–1279, 2013.
Collins, A. M., N. J. V. Skaer, T. Gheysens, D. Knight, C. Bertram, H. I. Roach, R. O. C. Oreffo, S. Von-Aulock, T. Baris, J. Skinner, and S. Mann. Bone-like resorbable silk-based scaffolds for load-bearing osteoregenerative applications. Adv. Mater. 21:75–78, 2009.
Conoscenti, G., V. L. Carrubba, and V. Brucato. A versatile technique to produce porous polymeric Scaffolds: The thermally induced phase separation (TIPS) Method. Arch. Chem. Res. 01:1–3, 2017.
Conoscenti, G., T. Schneider, K. Stoelzel, F. CarfiPavia, V. Brucato, C. Goegele, V. La Carrubba, and G. Schulze-Tanzil. PLLA scaffolds produced by thermally induced phase separation (TIPS) allow human chondrocyte growth and extracellular matrix formation dependent on pore size. Mater. Sci. Eng. C 80:449–459, 2017.
Cornell, C. N., J. M. Lane, M. Chapman, R. Merkow, D. Seligson, S. Henry, R. Gustilo, and K. Vincent. Multicenter trial of Collagraft as bone graft substitute. J. Orthop. Trauma 5:1–8, 1991.
Costantini, M., and A. Barbetta. Gas foaming technologies for 3D scaffold engineering. Funct. Tissue Eng. Scaffolds 5:127–149, 2018.
Crockett, J. C., D. J. Mellis, D. I. Scott, and M. H. Helfrich. New knowledge on critical osteoclast formation and activation pathways from study of rare genetic diseases of osteoclasts: focus on the RANK/RANKL axis. Osteoporos. Int. 22:1–20, 2011.
Crovace, A. M., A. D. Giancamillo, F. Gervaso, L. Mangiavini, D. Zani, F. Scalera, B. Palazzo, D. Izzo, M. Agnoletto, M. Domenicucci, C. Sosio, A. Sannino, M. D. Giancamillo, and G. M. Peretti. Evaluation of in vivo response of three biphasic scaffolds for osteochondral tissue regeneration in a sheep model. Vet. Sci. 6:90, 2019.
Cui, X., T. Boland, D. D. D’Lima, and M. K. Lotz. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat. Drug Deliv. Formul. 6:149–155, 2012.
Dababneh, A. B., and I. T. Ozbolat. Bioprinting technology: A current state-of-the-art review. J. Manufactur. Sci. Eng. Trans. ASME 136:6, 2014.
Dallas, S. L., and L. F. Bonewald. Dynamics of the transition from osteoblast to osteocyte. Skeletal Biol. Med. 1192:437–443, 2010.
Damsky, C. H. Extracellular matrix–integrin interactions in osteoblast function and tissue remodeling. Bone 25:95–96, 1999.
Darus, F., and M. Jaafar. Enhancement of carbonate apatite scaffold properties with surface treatment and alginate and gelatine coating. J. Porous Mater. 27:831–842, 2020.
Di Martino, A., M. Sittinger, and M. V. Risbud. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 26:5983–5990, 2005.
Donnaloja, F., E. Jacchetti, M. Soncini, and M. T. Raimondi. Natural and synthetic polymers for bone scaffolds optimization. Polymers 12:905, 2020.
dos Santos, D. M., D. S. Correa, E. S. Medeiros, J. E. Oliveira, and L. H. C. Mattoso. Advances in functional polymer nanofibers: From spinning fabrication techniques to recent biomedical applications. ACS Appl. Mater. Interfaces 12:45673–45701, 2020.
Drzewiecki, K. E., A. S. Parmar, I. D. Gaudet, J. R. Branch, D. H. Pike, V. Nanda, and D. I. Shreiber. Methacrylation induces rapid, temperature-dependent, reversible self-assembly of type-I collagen. Langmuir 30:11204–11211, 2014.
Duan, B., E. Kapetanovic, L. A. Hockaday, and J. T. Butcher. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 10:1836–1846, 2014.
Dzobo, K., N. E. Thomford, D. A. Senthebane, H. Shipanga, A. Rowe, C. Dandara, M. Pillay, and K. S. C. M. Motaung. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018. https://doi.org/10.1155/2018/2495848.
Einhorn, T. A., and L. C. Gerstenfeld. Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 11:45–54, 2015.
Elbadawi, M., J. Meredith, L. Hopkins, and I. Reaney. Progress in bioactive metal and ceramic implants for load-bearing application. Adv. Tech. Bone Regener. 2016. https://doi.org/10.5772/62598.
Eltom, A., G. Zhong, and A. Muhammad. Scaffold techniques and designs in tissue engineering functions and purposes: A review. Adv. Mater. Sci. Eng. 2019:1–13, 2019.
Everts, V., J. M. Delaisse, W. Korper, D. C. Jansen, W. Tigchelaar-Gutter, P. Saftig, and W. Beertsen. The bone lining cell: Its role in cleaning Howship’s lacunae and initiating bone formation. J. Bone Miner. Res. 17:77–90, 2002.
Fernandez de Grado, G., L. Keller, Y. Idoux-Gillet, Q. Wagner, A.-M. Musset, N. Benkirane-Jessel, F. Bornert, and D. Offner. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 9:2041731418776819, 2018.
Florencio-Silva, R., G. R. D. Sasso, E. Sasso-Cerri, M. J. Simoes, and P. S. Cerri. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res. Int. 2015. https://doi.org/10.1155/2015/421746.
Gan, D. L., M. Liu, T. Xu, K. F. Wang, H. Tan, and X. Lu. Chitosan/biphasic calcium phosphate scaffolds functionalized with BMP-2-encapsulated nanoparticles and RGD for bone regeneration. J. Biomed. Mater. Res. Part A 106:2613–2624, 2018.
Gao, C. D., Y. W. Deng, P. Feng, Z. Z. Mao, P. J. Li, B. Yang, J. J. Deng, Y. Y. Cao, C. J. Shuai, and S. P. Peng. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int. J. Mol. Sci. 15:4714–4732, 2014.
Gay, S., G. Lefebvre, M. Bonnin, B. Nottelet, F. Boury, A. Gibaud, and B. Calvignac. PLA scaffolds production from Thermally Induced Phase Separation: Effect of process parameters and development of an environmentally improved route assisted by supercritical carbon dioxide. J. Supercrit. Fluids 136:123–135, 2018.
Ghassemi, T., A. Shahroodi, M. H. Ebrahimzadeh, A. Mousavian, J. Movaffagh, and A. Moradi. Current concepts in scaffolding for bone tissue engineering. Arch. Bone Jt. Surg. 6:90–99, 2018.
Glass, D. A., P. Bialek, J. D. Ahn, M. Starbuck, M. S. Patel, H. Clevers, M. M. Taketo, F. X. Long, A. P. McMahon, R. A. Lang, and G. Karsenty. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 8:751–764, 2005.
Grottkau, B. E., Z. Hui, Y. Yao, and Y. Pang. Rapid fabrication of anatomically-shaped bone scaffolds using indirect 3D printing and perfusion techniques. Int. J. Mol. Sci. 21:315, 2020.
Guillemot, F., A. Souquet, S. Catros, B. Guillotin, J. Lopez, M. Faucon, B. Pippenger, R. Bareille, M. Remy, S. Bellance, P. Chabassier, J. C. Fricain, and J. Amedee. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 6:2494–2500, 2010.
Guillotin, B., A. Souquet, S. Catros, M. Duocastella, B. Pippenger, S. Bellance, R. Bareille, M. Remy, L. Bordenave, J. Amedee, and F. Guillemot. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 31:7250–7256, 2010.
Hench, L. L., and J. M. Polak. Third-generation biomedical materials. Science 295:1014, 2002.
Huang, W., X. Li, X. Shi, and C. Lai. Microsphere based scaffolds for bone regenerative applications. Biomater. Sci. 2:1145, 2014.
C.W. Hull, Apparatus for production of three-dimensional objects by stereolithography, https://patents.google.com/patent/US4575330A/en, 3D Systems Inc, USA, 1984.
Jakus, A. E., A. L. Rutz, S. W. Jordan, A. Kannan, S. M. Mitchell, C. Yun, K. D. Koube, S. C. Yoo, H. E. Whiteley, C.-P. Richter, R. D. Galiano, W. K. Hsu, S. R. Stock, E. L. Hsu, and R. N. Shah. Hyperelastic “bone”: A highly versatile, growth factor–free, osteoregenerative, scalable, and surgically friendly biomaterial. Sci. Transl. Med. 8:358, 2016.
Ji, C. D., N. Annabi, M. Hosseinkhani, S. Sivaloganathan, and F. Dehghani. Fabrication of poly-(dl)-lactide/polyethylene glycol scaffolds using the gas foaming technique. Acta Biomater. 8:570–578, 2012.
Jimi, E., S. Hirata, K. Osawa, M. Terashita, C. Kitamura, and H. Fukushima. The current and future therapies of bone regeneration to repair bone defects. Int. J. Dent. 2012:148261, 2012.
Jose, G., K. T. Shalumon, H.-T. Liao, C.-Y. Kuo, and J.-P. Chen. Preparation and characterization of surface heat sintered nanohydroxyapatite and nanowhitlockite embedded poly (lactic-co-glycolic acid) microsphere bone graft scaffolds. Vitro and in vivo studies. Int. J. Mol. Sci. 21:528, 2020.
Jun, I., H.-S. Han, J. Edwards, and H. Jeon. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 19:745, 2018.
Kaliva, M., A. Georgopoulou, D. A. Dragatogiannis, C. A. Charitidis, M. Chatzinikolaidou, and M. Vamvakaki. Biodegradable chitosan-graft-poly(l-lactide) copolymers for bone tissue engineering. Polymers 12:316, 2020.
Kim, S., Z.-K. Cui, J. Fan, A. Fartash, T. L. Aghaloo, and M. Lee. Photocrosslinkable chitosan hydrogels functionalized with the RGD peptide and phosphoserine to enhance osteogenesis. J. Mater. Chem. B 4:5289–5298, 2016.
Kim, M. S., and G. Kim. Three-dimensional electrospun polycaprolactone (PCL)/alginate hybrid composite scaffolds. Carbohydr. Polym. 114:213–221, 2014.
Kokubo, T., H. M. Kim, M. Kawashita, and T. Nakamura. REVIEW Bioactive metals: Preparation and properties. J. Mater. Sci. Mater. Med. 15:99–107, 2004.
Kundu, B., N. E. Kurland, S. Bano, C. Patra, F. B. Engel, V. K. Yadavalli, and S. C. Kundu. Silk proteins for biomedical applications: Bioengineering perspectives. Prog. Polym. Sci. 39:251–267, 2014.
Kundu, J., F. Pati, J. H. Shim, and D. W. Cho. Rapid prototyping technology for bone regeneration. Rapid Prototyp. Biomater. 70:254–284, 2014.
Lee, D. J., S. Diachina, Y. T. Lee, L. Zhao, R. Zou, N. Tang, H. Han, X. Chen, and C.-C. Ko. Decellularized bone matrix grafts for calvaria regeneration. J. Tissue Eng. 7:2041731416680306, 2016.
Li, J. P., M. J. Chen, X. Q. Fan, and H. F. Zhou. Recent advances in bioprinting techniques: approaches, applications and future prospects. J. Transl. Med. 14:1–15, 2016.
Li, Y., S.-K. Chen, L. Li, L. Qin, X.-L. Wang, and Y.-X. Lai. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 3:95–104, 2015.
Li, J. J., K. Kim, S. I. Roohani-Esfahani, J. Guo, D. L. Kaplan, and H. Zreiqat. A biphasic scaffold based on silk and bioactive ceramic with stratified properties for osteochondral tissue regeneration. J. Mater. Chem. B 3:5361–5376, 2015.
Li, Q., T. Wang, G.-F. Zhang, X. Yu, J. Zhang, G. Zhou, and Z.-H. Tang. A Comparative evaluation of the mechanical properties of two calcium phosphate/collagen composite materials and their osteogenic effects on adipose-derived stem cells. Stem Cells Int. 2016:1–12, 2016.
Lin, Y. X., Z. Y. Ding, X. B. Zhou, S. T. Li, M. Xie, Z. Z. Li, and G. D. Sun. In vitro and in vivo evaluation of the developed PLGA/HAp/Zein scaffolds for bone-cartilage interface regeneration. Biomed. Environ. Sci. 28:1–12, 2015.
Lin, Y., M. Umebayashi, M.-N. Abdallah, G. Dong, M. G. Roskies, Y. F. Zhao, M. Murshed, Z. Zhang, and S. D. Tran. Combination of polyetherketoneketone scaffold and human mesenchymal stem cells from temporomandibular joint synovial fluid enhances bone regeneration. Sci. Rep. 2019. https://doi.org/10.1038/s41598-018-36778-2.
Liu, M., and Y. Lv. Reconstructing bone with natural bone graft: A review of in vivo studies in bone defect animal model. Nanomaterials 8:999, 2018.
Loordhuswamy, A., S. Thinakaran, and G. D. Venkateshwapuram Rangaswamy. Centrifugal spun osteoconductive ultrafine fibrous mat as a scaffold for bone regeneration. J. Drug Deliv. Sci. Technol. 60:101978, 2020.
Lu, J., and X. Wang. Biomimetic self-assembling peptide hydrogels for tissue engineering applications. Biomimetic Med. Mater. 1064:297–312, 2018.
Manassero, M., A. Decambron, N. Guillemin, H. Petite, R. Bizios, and V. Viateau. Coral scaffolds in bone tissue engineering and bone regeneration. Cnidaria: Past Present Future, pp. 691–714, 2016.
Mandrycky, C., Z. J. Wang, K. Kim, and D. H. Kim. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 34:422–434, 2016.
Marelli, B., C. E. Ghezzi, A. Alessandrino, J. E. Barralet, G. Freddi, and S. N. Nazhat. Silk fibroin derived polypeptide-induced biomineralization of collagen. Biomaterials 33:102–108, 2012.
Martin, R. B., D. B. Burr, N. A. Sharkey, and D. P. Fyhrie. Mechanical Properties of Bone. Skeletal Tissue Mech. 12:355–422, 2015.
Maude, S., E. Ingham, and A. Aggeli. Biomimetic self-assembling peptides as scaffolds for soft tissue engineering. Nanomedicine 8:823–847, 2013.
McGovern, J. A., M. Griffin, and D. W. Hutmacher. Animal models for bone tissue engineering and modelling disease. Dis. Models Mech. 11:4, 2018.
Meinel, L., S. Hofmann, V. Karageorgiou, C. Kirker-Head, J. McCool, G. Gronowicz, L. Zichner, R. Langer, G. Vunjak-Novakovic, and D. L. Kaplan. The inflammatory responses to silk films in vitro and in vivo. Biomaterials 26:147–155, 2005.
Moest, T., K. A. Schlegel, M. Kesting, M. Fenner, R. Lutz, D. M. Beck, E. Nkenke, and C. von Wilmowsky. A new standardized critical size bone defect model in the pig forehead for comparative testing of bone regeneration materials. Clin. Oral Investig. 1:1–11, 2019.
Montero, F. E., R. A. Rezende, J. V. L. da Silva, and M. A. Sabino. Development of a smart bioink for bioprinting applications. Front. Mech. Eng. 5:56, 2019.
Morgan, E. F., G. U. Unnikrisnan, and A. I. Hussein. Bone mechanical properties in healthy and diseased states. Annu. Rev. Biomed. Eng. 20:119–143, 2018.
Motamedian, S. R., S. Hosseinpour, M. G. Ahsaie, and A. Khojasteh. Smart scaffolds in bone tissue engineering: A systematic review of literature. World J. Stem Cells 7:657–668, 2015.
Murata, M., F. Maki, D. Sato, T. Shibata, and M. Arisue. Bone augmentation by onlay implant using recombinant human BMP-2 and collagen on adult rat skull without periosteum. Clin. Oral Implant Res. 11:289–295, 2000.
Muzzarelli, R. A. A., M. Mattioli-Belmonte, C. Tietz, R. Biagini, G. Ferioli, M. A. Brunelli, M. Fini, R. Giardino, P. Ilari, and G. Biagini. Stimulatory effect on bone formation exerted by a modified chitosan. Biomaterials 15:1075–1081, 1994.
Navarro, M., A. Michiardi, O. Castano, and J. A. Planell. Biomaterials in orthopaedics. J. R. Soc. Interface 5:1137–1158, 2008.
Nguyen, L. H., N. Annabi, M. Nikkhah, H. Bae, L. Binan, S. Park, Y. Q. Kang, Y. Z. Yang, and A. Khademhosseini. Vascularized bone tissue engineering: Approaches for potential improvement. Tissue Eng. Part B 18:363–382, 2012.
Nguyen, T. B. L., and B. T. Lee. A combination of biphasic calcium phosphate scaffold with hyaluronic acid-gelatin hydrogel as a new tool for bone regeneration. Tissue Eng. Part A 20:1993–2004, 2014.
O’Brien, F. J. Biomaterials & scaffolds for tissue engineering. Mater. Today 14:88–95, 2011.
Orwoll, E. S. Clinical practice guidelines for osteoporosis: Translating data to patients? Ann. Intern. Med. 166:852–853, 2017.
Pamula, E., J. Kokoszka, K. Cholewa-Kowalska, M. Laczka, L. Kantor, L. Niedzwiedzki, G. C. Reilly, J. Filipowska, W. Madej, M. Kolodziejczyk, G. Tylko, and A. M. Osyczka. Degradation, bioactivity, and osteogenic potential of composites made of PLGA and two different sol–gel bioactive glasses. Ann. Biomed. Eng. 39:2114–2129, 2011.
Pati, F., J. Jang, J. W. Lee, and D.-W. Cho. Extrusion Bioprinting, Essentials of 3D Biofabrication and Translation. Boca Raton: Academic Press, pp. 123–152, 2015.
Phipps, M. C., W. C. Clem, J. M. Grunda, G. A. Dines, and S. L. Bellis. Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration. Biomaterials 33:524–534, 2012.
Policastro, G. M., F. Lin, L. A. SmithCallahan, A. Esterle, M. Graham, K. SloanStakleff, and M. L. Becker. OGP functionalized phenylalanine-based poly(ester urea) for enhancing osteoinductive potential of human mesenchymal stem cells. Biomacromolecules 16:1358–1371, 2015.
Polo-Corrales, L., M. Latorre-Esteves, and J. E. Ramirez-Vick. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 14:15–56, 2014.
Prakash, D., and D. Learmonth. Natural progression of osteo-chondral defect in the femoral condyle. Knee 9:7–10, 2002.
Qaseem, A., M. A. Forciea, R. M. McLean, T. D. Denberg, and C. G. C. A. Coll. Treatment of low bone density or osteoporosis to prevent fractures in men and women: A clinical practice guideline update from the American College of Physicians. Ann. Internal Med. 166:818, 2017.
Qu, H., H. Fu, Z. Han, and Y. Sun. Biomaterials for bone tissue engineering scaffolds: a review. RSC Adv. 9:26252–26262, 2019.
Randall, I. Liquid-in-liquid printing method could put 3D-printed organs in reach. Science 2019. https://doi.org/10.1126/science.aba0750.
Ratnayake, J. T. B., M. Mucalo, and G. J. Dias. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B 105:1285–1299, 2017.
Rezwan, K., Q. Z. Chen, J. J. Blaker, and A. R. Boccaccini. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 27:3413–3431, 2006.
Rhee, S., J. L. Puetzer, B. N. Mason, C. A. Reinhart-King, and L. J. Bonassar. 3D bioprinting of spatially heterogeneous collagen constructs for cartilage tissue engineering. ACS Biomater. Sci. Eng. 2:1800–1805, 2016.
Ripamonti, U. The morphogenesis of bone in replicas of porous hydroxyapatite obtained from conversion of calcium-carbonate exoskeletons of coral. J. Bone Joint Surg. Am. 73:692–703, 1991.
Rodel, M., K. Baumann, J. Groll, and U. Gbureck. Simultaneous structuring and mineralization of silk fibroin scaffolds. J. Tissue Eng. 9:2041731418788509, 2018.
Rodriguez-Salvador, M., R. M. Rio-Belver, and G. Garechana-Anacabe. Scientometric and patentometric analyses to determine the knowledge landscape in innovative technologies: The case of 3D bioprinting. PLoS ONE 12:e0180375, 2017.
Roseti, L., V. Parisi, M. Petretta, C. Cavallo, G. Desando, I. Bartolotti, and B. Grigolo. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 78:1246–1262, 2017.
Ruehe, B., S. Niehues, S. Heberer, and K. Nelson. Miniature pigs as an animal model for implant research: bone regeneration in critical-size defects. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 108:699–706, 2009.
Saleem, M., S. Rasheed, and C. Yougen. Silk fibroin/hydroxyapatite scaffold: A highly compatible material for bone regeneration. Sci. Technol. Adv. Mater. 21:242–266, 2020.
Saravanan, S., R. S. Leena, and N. Selvamurugan. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 93:1354–1365, 2016.
Scaglione, S., P. Giannoni, P. Bianchini, M. Sandri, R. Marotta, G. Firpo, U. Valbusa, A. Tampieri, A. Diaspro, P. Bianco, and R. Quarto. Order versus Disorder: In vivo bone formation within osteoconductive scaffolds. Sci. Rep. 2:274, 2012.
Schaffler, M. B., W. Y. Cheung, R. Majeska, and O. Kennedy. Osteocytes: Master orchestrators of bone. Calcif. Tissue Int. 94:5–24, 2014.
Seol, Y. J., J. Y. Lee, Y. J. Park, Y. M. Lee, K. Young, I. C. Rhyu, S. J. Lee, S. B. Han, and C. P. Chung. Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnol. Lett. 26:1037–1041, 2004.
Shi, C., Z. Yuan, F. Han, C. Zhu, and B. Li. Polymeric biomaterials for bone regeneration. Ann. Jt. 1:27–27, 2016.
Singh, M. R., S. Patel, and D. Singh. Natural polymer-based hydrogels as scaffolds for tissue engineering. Nanobiomater. Soft Tissue Eng. 5:231–260, 2016.
Sohn, H. S., and J. K. Oh. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 23:9, 2019.
Sokolova, V., K. Kostka, K. T. Shalumon, O. Prymak, J.-P. Chen, and M. Epple. Synthesis and characterization of PLGA/HAP scaffolds with DNA-functionalised calcium phosphate nanoparticles for bone tissue engineering. J. Mater. Sci. 31:1–12, 2020.
Song, J.-L., X.-Y. Fu, A. Raza, N.-A. Shen, Y.-Q. Xue, H.-J. Wang, and J.-Y. Wang. Enhancement of mechanical strength of TCP-alginate based bioprinted constructs. J. Mech. Behav. Biomed. Mater. 103:103533, 2020.
Soysa, N. S., N. Alles, K. Aoki, and K. Ohya. Osteoclast formation and differentiation: An overview. J Med Dent Sci 59:65–74, 2012.
Su, A., and S. J. Al’Aref. History of 3D Printing, 3D Printing Applications in Cardiovascular Medicine. Boca Raton: Academic Press, pp. 1–10, 2018.
Subramaniam, S., Y. H. Fang, S. Sivasubramanian, F. H. Lin, and C. P. Lin. Hydroxyapatite-calcium sulfate-hyaluronic acid composite encapsulated with collagenase as bone substitute for alveolar bone regeneration. Biomaterials 74:99–108, 2016.
Tamay, D. G., T. DursunUsal, A. S. Alagoz, D. Yucel, N. Hasirci, and V. Hasirci. 3D and 4D printing of polymers for tissue engineering applications. Front. Bioeng. Biotechnol. 7:164, 2019.
Tamay, D. G., T. D. Usal, A. S. Alagoz, D. Yucel, N. Hasirci, and V. Hasirci. 3D and 4D printing of polymers for tissue engineering applications. Front. Bioeng. Biotechnol. 7:164, 2019.
Tan, Z., C. Parisi, L. Di Silvio, D. Dini, and A. E. Forte. Cryogenic 3D printing of super soft hydrogels. Sci. Rep. 7:16293, 2017.
Tarafder, S., and S. Bose. Polycaprolactone-coated 3D printed tricalcium phosphate scaffolds for bone tissue engineering, vitro alendronate release behavior and local delivery effect on in vivo osteogenesis. ACS Appl. Mater. Interfaces 6:9955–9965, 2014.
Teixeira, S., H. Fernandes, A. Leusink, C. van Blitterswijk, M. P. Ferraz, F. J. Monteiro, and J. de Boer. In vivo evaluation of highly macroporous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 93:567–575, 2010.
Thadavirul, N., P. Pavasant, and P. Supaphol. Development of polycaprolactone porous scaffolds by combining solvent casting, particulate leaching, and polymer leaching techniques for bone tissue engineering. J. Biomed. Mater. Res. Part A 102(10):3379–3392, 2013.
Thanh, D. T. M., P. T. T. Trang, N. T. Thom, N. T. Phuong, P. T. Nam, N. T. T. Trang, J. Seo-Park, and T. Hoang. Effects of porogen on structure and properties of poly lactic acid/hydroxyapatite nanocomposites (PLA/HAp). J. Nanosci. Nanotechnol. 16:9450–9459, 2016.
Turco, G., E. Marsich, F. Bellomo, S. Semeraro, I. Donati, F. Brun, M. Grandolfo, A. Accardo, and S. Paoletti. Alginate/hydroxyapatite biocomposite for bone ingrowth: A trabecular structure with high and isotropic connectivity. Biomacromolecules 10:1575–1583, 2009.
Turnbull, G., J. Clarke, F. Picard, P. Riches, L. Jia, F. Han, B. Li, and W. Shu. 3D bioactive composite scaffolds for bone tissue engineering. Bioactive Mater. 3:278–314, 2018.
Valot, L., J. Martinez, A. Mehdi, and G. Subra. Chemical insights into bioinks for 3D printing. Chem. Soc. Rev. 48:4049–4086, 2019.
Velasco, M. A., C. A. Narváez-Tovar, and D. A. Garzón-Alvarado. Design, materials, and mechanobiology of biodegradable scaffolds for bone tissue engineering. Biomed. Res. Int. 2015:1–21, 2015.
Venkatesan, J., and S. K. Kim. Chitosan composites for bone tissue engineering-an overview. Marine Drugs 8:2252–2266, 2010.
Wang, M. M., R. L. Flores, L. Witek, A. Torroni, A. Ibrahim, Z. Wang, H. A. Liss, B. N. Cronstein, C. D. Lopez, S. G. Maliha, and P. G. Coelho. Dipyridamole-loaded 3D-printed bioceramic scaffolds stimulate pediatric bone regeneration in vivo without disruption of craniofacial growth through facial maturity. Sci. Rep. 9:1–15, 2019.
Wang, C., J. Tanjaya, J. Shen, S. Lee, B. Bisht, H. C. Pan, S. Pang, Y. Zhang, E. A. Berthiaume, E. Chen, A. L. Da Lio, X. Zhang, K. Ting, S. Guo, and C. Soo. Peroxisome proliferator-activated receptor-γ knockdown impairs bone morphogenetic protein-2-induced critical-size bone defect repair. Am. J. Pathol. 189:648–664, 2019.
Weiss, L. E., C. H. Amon, S. Finger, E. D. Miller, D. Romero, I. Verdinelli, L. M. Walker, and P. G. Campbell. Bayesian computer-aided experimental design of heterogeneous scaffolds for tissue engineering. Comput. Aided Des. 37:1127–1139, 2005.
Wijshoff, H. The dynamics of the piezo inkjet printhead operation. Phys. Rep. 491:77–177, 2010.
Wilkinson, D. C., J. A. Alva-Ornelas, J. M. S. Sucre, P. Vijayaraj, A. Durra, W. Richardson, S. J. Jonas, M. K. Paul, S. Karumbayaram, B. Dunn, and B. N. Gomperts. Development of a three-dimensional bioengineering technology to generate lung tissue for personalized disease modeling. Stem Cells Transl. Med. 6:622–633, 2017.
Woodruff, M. A., C. Lange, J. Reichert, A. Berner, F. L. Chen, P. Fratzl, J. T. Schantz, and D. W. Hutmacher. Bone tissue engineering: from bench to bedside. Mater. Today 15:430–435, 2012.
Xu, T., J. Jin, C. Gregory, J. J. Hickman, and T. Boland. Inkjet printing of viable mammalian cells. Biomaterials 26:93–99, 2005.
Xu, C. X., P. Q. Su, X. F. Chen, Y. C. Meng, W. H. Yu, A. P. Xiang, and Y. J. Wang. Biocompatibility and osteogenesis of biomimetic bioglass–collagen–phosphatidylserine composite scaffolds for bone tissue engineering. Biomaterials 32:1051–1058, 2011.
Xu, H. H. K., L. Zhao, and M. D. Weir. Stem cell-calcium phosphate constructs for bone engineering. J. Dent. Res. 89:1482–1488, 2010.
Xu, T., W. X. Zhao, J. M. Zhu, M. Z. Albanna, J. J. Yoo, and A. Atala. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials 34:130–139, 2013.
Yang, H., N. Hong, H. Liu, J. Wang, Y. Li, and S. Wu. Differentiated adipose-derived stem cell cocultures for bone regeneration in RADA16-I in vitro. J. Cell. Physiol. 233:9458–9472, 2018.
Yuan, N., K. S. Rezzadeh, and J. C. Lee. Biomimetic scaffolds for osteogenesis. Receptors Clin. Investig. 2:3, 2015.
Zaky, S. H., and R. Cancedda. Engineering craniofacial structures: Facing the challenge. J. Dent. Res. 88:1077–1091, 2009.
Zhai, P., X. Peng, B. Li, Y. Liu, H. Sun, and X. Li. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 151:1224–1239, 2020.
Zhang, D., X. Wu, J. Chen, and K. Lin. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 3:129–138, 2018.
Zhao, F., C. Fu, H. Bai, J. Zhu, Z. Niu, Y. Wang, J. Li, X. Yang, and Y. Bai. Enhanced cell proliferation and osteogenic differentiation in electrospun PLGA/hydroxyapatite nanofibre scaffolds incorporated with graphene oxide. PLoS ONE 12:e0188352, 2017.
Zhu, N., and X. Che. Biofabrication of tissue scaffolds. Adv. Biomater. Sci. Biomed. Appl. 1:11, 2013. https://doi.org/10.5772/54125.
Zhu, M. L., S. Lin, Y. X. Sun, Q. Feng, G. Li, and L. M. Bian. Hydrogels functionalized with N-cadherin mimetic peptide enhance osteogenesis of hMSCs by emulating the osteogenic niche. Biomaterials 77:44–52, 2016.
Zilberman, M. Active implants and scaffolds for tissue regeneration. Active Implants Scaffolds Tissue Regener. 8:1–514, 2011.
Acknowledgments
M.P. acknowledges Prof. Steven Dubinett, Prof. Brigitte Gomperts and Prof. Volker Hartenstein, from UCLA for providing constant support and mentoring. A.M. takes this opportunity to acknowledge Raktim Chattopadhyay of Esperer Onco Nutrition Pvt. Ltd. for his constant support. B.B. would like to acknowledge Prof. Jay M. Lee, Prof. Steven Dubinett, and Prof. Brigitte Gomperts, from UCLA for providing constant support and mentoring.
Author Contributions
Conceptualization, MP; writing - original draft preparation, BB, AH, AM, and MP, review and editing, MP.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bisht, B., Hope, A., Mukherjee, A. et al. Advances in the Fabrication of Scaffold and 3D Printing of Biomimetic Bone Graft. Ann Biomed Eng 49, 1128–1150 (2021). https://doi.org/10.1007/s10439-021-02752-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-021-02752-9