Abstract
The objective of this study was to describe the proton promoted disproportion of synthetic manganite (γ-MnOOH) and to characterise the resulting phase transformations. The solution and remaining solid phase after disproportionation was analysed by techniques including atomic absorbance spectroscopy, X-ray diffraction (XRD), atomic force microscopy (AFM) and scanning electron microscopy (SEM). In suspensions with pH between 5 and 7, −log[H+] was monitored for 17 months and equilibrium constants were determined at 9, 12 and 17 months of reaction time for the following reaction (25 °C, 0.1 M (Na)NO3):
The formed MnO2 ages with time and the equilibrium constant for a metastable phase (ramsdellite or nsutite) as well as the most stable phase, pyrolusite (β-MnO2), was determined. Furthermore, combined pH and pe (Eh) measurements were performed to study the equilibrium;
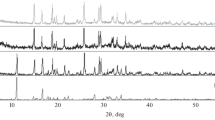
Real-time AFM measurements of the dissolution showed shrinkage of the length of the manganite needles with time (2 hours). After 1 week SEM images showed that this decreased length also was followed by a reduced thickness of the manganite needles. From the SEM images the morphology of the formed Mn(IV) oxides was studied. At pH 2.6, pyrolusite (β-MnO2) and MnCl2 were found in the XRD patterns. Throughout the pH range there were indications of ramsdellite (MnO1.97) in the XRD patterns, which coincided with the existence of a fraction of needle shaped crystals with smaller dimensions (compared to manganite) in the SEM images. These observations together with the long term dissolution experiments suggest that the dissolution of manganite initially forms a ramsdellite or nsutite phase that over time rearranges to form pyrolusite.
Similar content being viewed by others
References
J. Ambrose A. K. Covington H. R. Thirsk (1969) ArticleTitleStandard electrode potential of β-Manganese Dioxide and its relation to other properties Trans. Faraday Soc. 65 1897–1905 Occurrence Handle10.1039/tf9696501897
M. Amouric S. Parc D. Nahon (1991) ArticleTitleHigh resolution transmission electron microscopy study of Mn-hydroxide transformations and accompanying phases in a lateric profile of Moanda, Gabon Clays and Clay Minerals 39 IssueID3 254–263
Berry L. G. and Mason B. (1983) Mineralogy – Concepts, Descriptions, Determinations, 2nd Edn. (ed. R. V. Dietrich), W. H. Freeman and Company, San Fransisco.
L. Bochatay P. Persson S. Sjöberg (2000) ArticleTitleMetal ion coordination at the water-manganite (γ-MnOOH) interface I. An EXAFS study of Cadmium(II) J. Colloid Interface Sci. 229 584–592
O. Bricker (1965) ArticleTitleSome stability relations in the system Mn–O2–H2O at 25° and one atmosphere total pressure Am. Mineral. 50 1296–1354
S. Brunauer P. H. Emmett E. Teller (1938) ArticleTitleAdsorption of gases in multimolecular layers J. Am. Chem. Soc. 60 309–319 Occurrence Handle10.1021/ja01269a023
Y. Chabre J. Pannetier (1995) ArticleTitleStructural and electrochemical properties of the proton/γ-MnO2 system Prog. Solid. St. Chem. 23 1–130 Occurrence Handle10.1016/0079-6786(94)00005-2
Q. Chiu Particlevan J. G. Hering (2000) ArticleTitleArsenic adsorption and oxidation at manganite surfaces. 1. Method for simultaneous determination of adsorbed and dissolved arsenic species Environ. Sci. Technol. 34 2029–2034 Occurrence Handle10.1021/es990788p
Eriksson G. (1979) An algorithm for the computation of aqueous multi-component, multiphase equilibria. Anal. Chim. Acta 112, 375–383. http://www.chem.umu.se/dep/inorgchem/samarbeta/WinSGW_eng.stm
G. Gattow von O. Glemser (1961) ArticleTitleDarstellung und Eigenschaften von Braunsteinen. III (Die ɛ-, β-, und α-Gruppe der Braunsteine, über Ramsdellit und über die Umwandlungen der Braunsteine Z. Anorg. Allg. Chemie. 309 121–150
R. Giovanoli U. Leuenberger (1969) ArticleTitleÜber die oxidation von manganoxidhydroxide Helv. Chim. Acta 52 2333–2347 Occurrence Handle10.1002/hlca.19690520815
L. Gunneriusson S. Sjöberg (1993) ArticleTitleSurface complexation in the H+-Goethite (α-FeOOH)-Hg(II)-chloride system J. Colloid Interface Sci. 156 121–128 Occurrence Handle10.1006/jcis.1993.1090
J. D. Hem C. J. Lind (1983) ArticleTitleNonequilibrium models for predicting forms of precipitated manganese oxides Geochim. Cosmochim. Acta 47 2037–2046 Occurrence Handle10.1016/0016-7037(83)90219-3
E. Högfeldt (1982) Stability Constants of Metal-ion Complexes – Part A: Inorganic Ligands Pergamon Press Oxford, England
InstitutionalAuthorNameICDD International Centre for Diffraction Data (1997) Powder Diffraction File Pennsylvania USA
C. A. Johnson A. G. Xyla (1991) ArticleTitleThe oxidation of chromium(III) to chromium(VI) on the surface of manganite (γ-MnOOH) Geochim. Cosmochim. Acta 55 2861–2866
J. K. Klewicki J. J. Morgan (1999) ArticleTitleDissolution of β-MnOOH particles by ligands: Pyrophosphate, etylenediaminetetraacetate, and citrate Geochim. Cosmochim. Acta 63 3017–3024 Occurrence Handle10.1016/S0016-7037(99)00229-X
T. Kohler T. Armbruster E. Libowitzky (1997) ArticleTitleHydrogen bonding and Jahn-Teller distortion in Groutite, α-MnOOH, and Manganite, γ-MnOOH, and their relations to the manganese dioxides ramsdellite and pyrolusite J. Solid St. Chem. 133 486–500 Occurrence Handle10.1006/jssc.1997.7516
W. L. Lindsay (1979) Chemical Equilibria in Soils John Wiley and Sons New York
A. Manceau V. A. Drits E. Silvester C. Bartoli B. Lanson (1997) ArticleTitleStructural mechanism of Co2+ oxidation by the phyllomanganate buserite Am. Mineral. 82 1150–1175
C. S. Mcardell A. T. Stone J. Tian (1998) ArticleTitleReaction of EDTA and realted aminocarboxylate chelating agents with CoIIIOOH (Heterogenite) and MnIIIOOH (Manganite) Environ. Sci.Technol. 32 2923–2930 Occurrence Handle10.1021/es980362v
J. W. Murray J. G. Dillard R. Giovanoli H. Moers W. Stumm (1985) ArticleTitleOxidation of Mn(II): Initial mineralogy, oxidation state and ageing Geochim. Cosmochim. Acta 49 463–470 Occurrence Handle10.1016/0016-7037(85)90038-9
P. S. Nico R. J. Zasoski (2001) ArticleTitleMn(III) center availability as a rate controlling factor in the oxidation of phenol and sulphide on δ-MnO2 Environ. Sci. Technol. 35 3338–3343 Occurrence Handle10.1021/es001848q
J. E. Post (1999) ArticleTitleManganese Oxide minerals: Crystal structures and economic and environmental significance Proc. Natl. Acad. Sci. USA 96 3447–3453
M. Ramstedt A. Shchukarev S. Sjöberg (2002) ArticleTitleCharacterization of hydrous manganite (γ-MnOOH) surfaces – an XPS study Surf. Interface Anal. 34 632–636 Occurrence Handle10.1002/sia.1376
M. Ramstedt B. Andersson A. Shchukarev S. Sjöberg (2004) ArticleTitleSurface properties of hydrous manganite (γ-MnOOH) – a potentiometric, electroacoustic and XPS study Langmuir 20 8224–8229 Occurrence Handle10.1021/la0496338
D. A. Shaughnessy H. Nitsche C. H. Booth D. K. Shuh G. A. Waychunas R. E. Wilson H. Gill K. J. Cantrell R. J. Serne (2003) ArticleTitleMolecular interfacial reactions between Pu(VI) and Manganese Oxide minerals Manganite and Hausmannite Environ. Sci. Technol. 37 3367–3374 Occurrence Handle10.1021/es025989z
L. G. Sillén A. E. Martell (1971) Stability Constants of Metal-ion Complexes Spec Publ. 25 The Chemical Society London
W. Stumm R. Giovanoli (1976) ArticleTitleOn the nature of particulate manganese in simulated lake waters Chimica 30 IssueID6 423–425
I. M. Varentsov G. Y. Grassely (1980) Geology and Geochemistry of Manganese, Volume 1 E. Schweizerbart’she Verlagsbuchhandlung Stuttgart, Germany
R. M. Weaver M. F. Hochella E. S. Ilton SuffixJr. (2002) ArticleTitleDynamic processes occurring at the Cr\(^{\rm III}_{\rm aq}\)-manganite (γ-MnOOH) interface: Simultaneous adsorption, microprecipitation, oxidation/reduction and dissolution Geochim. Cosmochim. Acta 66 4119–4132 Occurrence Handle10.1016/S0016-7037(02)00980-8
A. G. Xyla B. Sulzberger G. W. Luther SuffixIII. J. G. Hering P. Cappellen ParticleVan W. Stumm (1992) ArticleTitleReductive dissolution of the manganese(III,IV) (Hydr)oxides by oxalate: The effect of pH and light Langmuir 8 IssueID1 95–103 Occurrence Handle10.1021/la00037a019
N. Yamada M. Ohmasa (1986) ArticleTitleTextures in natural pyrolusite, β-MnO2, examined by 1 MV HRTEM Acta Cryst. B42 58–61
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramstedt, M., Sjöberg, S. Phase Transformations and Proton Promoted Dissolution of Hydrous Manganite (γ-MnOOH). Aquat Geochem 11, 413–431 (2005). https://doi.org/10.1007/s10498-005-7441-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10498-005-7441-2