Abstract
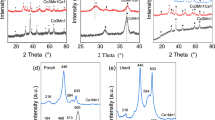
A series of Co–Cu composite oxides with different Co/Cu atomic ratios were prepared by a co-precipitation method. XRD, N2 sorption, TEM, XPS, H2-TPR, CO-TPR, CO-TPD and O2-TPD were used to characterize the structure and redox properties of the composite oxides. Only spinel structure of Co3O4 phase was confirmed for the Co–Cu composite oxides with Co/Cu ratios of 4/1 and 2/1, but the particle sizes of these composite oxides decreased evidently compared with Co3O4. These composite oxides could be reduced at lower temperatures than Co3O4 by either H2 or CO. CO and O2 adsorption amounts over the composite oxides were significantly higher than those over Co3O4. These results indicated a strong interaction between cobalt and copper species in the composite samples, possibly suggesting the formation of Cu x Co3−x O4 solid solution. For the preferential oxidation of CO in a H2-rich stream, the Co–Cu composite oxides (Co/Cu = 4/1–1/1) showed distinctly higher catalytic activities than both Co3O4 and CuO, and the formation of Cu x Co3−x O4 solid solution was proposed to contribute to the high catalytic activity of the composite catalysts. The Co–Cu composite oxide was found to exhibit higher catalytic activity than several other Co3O4-based binary oxides including Co–Ce, Co–Ni, Co–Fe and Co–Zn oxides.








Similar content being viewed by others
References
Steele BCH, Heinzel A (2001) Nature 414:345
Ratnasamy P, Srinivas D, Satyanarayana CVV, Manikandam P, Kumaran R, Senthil S, Sachin M, Shetti VN (2004) J Catal 221:455
Choudhary TV, Goodman DW (2002) Catal Today 77:65
Sanchez RMT, Ueda A, Tanaka K, Haruta M (1997) J Catal 168:125
Kahlich MJ, Gasteiger HA, Behm RJ (1997) J Catal 171:93
Son IH, Shamsuzzoha M (2002) J Catal 210:460
Pozdnyakova O, Tesehner D, Wootseh A, Kröhnert J, Steinhauer B, Sauer H, Toth L, Jentoft FC, KnoP-Gericke A, Paäl Z, Schlögl R (2006) J Catal 237:17
Tanaka K, Shou M, He H, Shi X (2006) Catal Lett 110:185
Iwasa N, Arai S, Arai M (2008) Appl Catal B 79:132
Teng Y, Sakurai H, Ueda A, Kobayashi T (1999) Int J Hydrogen Energy 24:355
Huang Y, Wang A, Li L, Wang X, Su D, Zhang T (2008) J Catal 255:144
Yung MM, Zhao ZK, Woods MP, Ozkan US (2008) J Mol Catal A 279:1
GuO Q, Liu Y (2007) React Kinet Catal Lett 92:19
GuO Q, Liu Y (2008) Appl Catal B 82:19
Moreno M, Baronetti GT, Laborde MA, Mariño FJ (2008) Int J Hydrogen Energy. doi:10.1016/j.ijhydene.2008.03.043
Luo MF, Ma JM, Lu JQ, Song YP, Wang YJ (2007) J Catal 246:52
Moretti E, Storaro L, Talon A, Patrono P, Pinzari F, Montanari T, Ramis G, Lenarda M (2008) Appl Catal A 344:165
Wang YZ, Zhao YX, Gao CG, Liu DS (2007) Catal Lett 116:136
Pollard MJ, Weinstock BA, Bitterwolf TE, Griffiths PR, Newbery AP, Paine III JB (2008) J Catal 254:218
Wang CB, Tang CW, Gau SJ, Chien SH (2005) Catal Lett 101:59
Mocuta C, Barbier A, Renaud G (2000) Appl Surf Sci 56:162
Mathew T, Shiju NR, Sreekumar K, Rao BS, Gopinath CS (2002) J Catal 210:405
Sreekumar K, Mathew T, Devassy BM, Rajagopal R, Vetrivel R, Rao BS (2001) Appl Catal 205:11
Sreekumar K, Mathew T, Rajagopal R, Vetrivel R, Rao BS (2000) Catal Lett 65:99
Said AA, Ai-Qasmi R (1996) Thermochim Acta 275:83
Zavyalova U, Nigrovski B, Pollok K, Langenhorst F, Müler B, Scholz P, Ondruschka B (2008) Appl Catal B 83:221
Porta P, Dragone R, Fierro G, Inversi M, Lo Jacono M, Moretti G (1991) J Mater Chem 1:311
Brunauer B, Emmett PH, Teller PHE (1938) J Am Chem Soc 60:309
Barrett EP, Joyner LS, Haienda PP (1951) J Am Chem Soc 73:373
Zarate RA, Hevia F, Fuentes S, Fuenzalida VM, Zúñiga A (2007) J Solid State Chem 180:1464
Cesar DV, Peréz CA, Schmal M, Salim VMM (2000) Appl Surf Sci 157:159
Dupin J-C, Gonbeau D, Vinatier P, Levasseur A (2000) Phys Chem Chem Phys 1319
Fierro G, Jacono ML, Inversi M, Dragone R, Porta P (2000) Top Catal 10:39
Acknowledgments
This work was supported by the National Natural Science Foundation of China (20773099), the National Basic Research Program of China (2005CB221408), the Key Scientific Project of Fujian Province of China (No. 2005HZ01-3), and the Program for New Century Excellent Talents in Fujian province (to Q. Zhang).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, D., Liu, X., Zhang, Q. et al. Cobalt and Copper Composite Oxides as Efficient Catalysts for Preferential Oxidation of CO in H2-Rich Stream. Catal Lett 127, 377–385 (2009). https://doi.org/10.1007/s10562-008-9693-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9693-0