Abstract
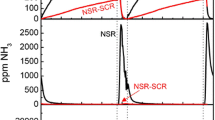
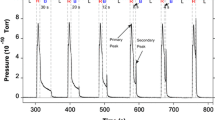
The effects of using NO or NO2 as the NO X source on the performance of a NO X storage/reduction catalyst were investigated from 200 to 500 °C. The evaluation included comparison with constant cycling times and trapping the same amount of NO X during the lean phase. With NO2 as the NO X source, better trapping and reduction performance was attained in comparison to NO, at all operating temperatures except 300 °C. This exception, under the conditions tested, was likely due to high NO oxidation activity and rapid trapping of NO2, although it is expected that extending the trapping time would lead to consistent differences. Several reasons for the observed improvements at 200, 400 and 500 °C with NO2 relative to NO are discussed. One that can explain the data, for both trapping and release improvement, is treating the monolith as an integral reactor. With NO2, more NO X is trapped at the very inlet of the catalyst, whereas with NO, the maximum in trapping during cycling occurs slightly downstream. Thus more of the catalyst can be used for trapping with NO2 as the NO X source. The decreased release during catalyst regeneration is similarly explained; with more being released at the very inlet, there is more residence time and therefore contact with downstream Pt sites, but more importantly more interaction between reductant and stored NO X . NH3 and N2O measurements support this conclusion.




Similar content being viewed by others
References
Bogner W, Kramer M, Krutzsch B, Pischinger S, Voigtlander D, Wenninger G, Wirbeleit F, Brogan M, Brisley R, Webster D (1995) Appl Catal B: Environ 7:153
Takahashi N, Shinjoh H, Iijima T, Suzuki T, Yamazaki K, Yokota K, Suzuki H, Miyoshi N, Matsumoto S, Tanizawa T, Tanaka T, Tateishi S, Kasahara K (1996) Catal Today 27:63
Hachisuka I, Hirata H, Ikeda Y, Matsumoto S (1999) SAE Technical Paper Series 1999-08-0571
Epling WS, Campbell LE, Yezerets A, Currier NW, Parks JE (2004) Catal Rev 46:163
Westerberg B, Fridell E (2001) J Mol Catal A: Chem 165:249
Kwak J, Kim D, Szailer T, Peden C, Szanyi J (2006) Catal Lett 111:3
Prinetto F, Ghiotti G, Nova I, Lietti L, Tronconi E, Forzatti P (2001) J Phys Chem B 105:12732
Toops T, Smith D, Epling WS, Parks J, Partridge W (2005) Appl Catal B: Environ 58:255
Jozsa P, Jobson E, Larsson M (2004) Top Catal 30/31:177
James D, Fourré E, Ishii M, Bowker M (2003) Appl Catal B: Environ 45:147
Poulston S, Rajaram RR (2003) Catal Today 81:603
Fridell E, Persson H, Westerberg B, Olsson L, Skoglundh M (2000) Catal Lett 66:71
Mahzoul H, Brilhac JF, Gilot P (1999) Appl Catal B: Environ 20:47
Nova I, Lietti L, Forzatti P (2008) Catal Today 136:128
Schmitz P, Baird R (2002) J Phys Chem B 106:4176
Hodjati S, Vaezzadeh K, Petit C, Pitchon V, Kiennemann A (2000) Catal Today 59:323
Erkfeldt S, Jobson E, Larsson M (2001) Top Catal 16/17:1
Fridell E, Skoglundh M, Westerberg B, Johansson S, Smedler G (1999) J Catal 183:196
Olsson L, Fridell E (2002) J Catal 210:340
Crocoll M, Kureti S, Weisweiler W (2005) J Catal 229:480
Mulla SS, Chen N, Cumaranatunge L, Delgass WN, Epling WS, Ribeiro FH (2006) Catal Today 114:57
Mulla SS, Chen N, Delgass WN, Epling WS, Ribeiro FH (2005) Catal Lett 100:3
Kabin K, Muncrief R, Harold M (2004) Catal Today 96:79
Rodrigues F, Juste L, Potvin C, Tempère JF, Blanchard G, Djéga-Mariadassou G (2001) Catal Lett 7:1
Meng L, Lin M, Fu Y, Hu T, Xie Y, Zhang J (2003) Top Catal 22:111
Lietti L, Forzatti P, Nova I, Tronconi E (2001) J Catal 204:175
Cant NW, Patterson MJ (2002) Catal Today 73:271
Epling WS, Parks JE, Campbell GC, Yezerets A, Currier NW, Campbell L (2004) Catal Today 96:21
Piacentini M, Maciejewski M, Baiker A (2005) Appl Catal B: Environ 60:265–275
Medhekar V, Balakotaiah V, Harold M (2007) Catal Today 121:226
Salasc S, Skoglundh M, Fridell E (2002) Appl Catal B: Environ 36:145
Kobayashi T, Yamada T, Kayano K, SAE Technical Paper Series 970745
Kikuyama S, Matsukuma I, Kikuchi R, Sasaki K, Eguchi K (2002) Appl Catal A: Gen 226:23
Laurent F, Pope C, Mahzoul H, Delfosse L, Gilot P (2003) Chem Eng Sci 58:1793
Aftab K, Mandur J, Budman H, Currier NW, Yezerets A, Epling WS (2008) Catal Lett 125:229
Olsson L, Westerberg B, Persson H, Fridell E, Skoglundh M, Andersson B (1999) J Phys Chem B 103:10433
Epling WS, Yezerets A, Currier NW (2006) Catal Lett 110:143
Choi J, Partridge WP, Epling WS, Currier NW, Yonushonis T (2006) Catal Today 114:102
Lindholm A, Currier NW, Fridell E, Yezerets A, Olsson L (2007) Appl Catal B: Environ 75:78
Cumaranatunge L, Mulla SS, Yezerets A, Currier NW, Delgass WN, Ribeiro FH (2007) J Catal 246:29
Pihl JA, Parks II JE, Daw CS, Root TW (2006) SAE Technical Paper Series 2006-01-3441
Kim DH, Chin Y-H, Kwak JH, Szanyi J, Peden CHF (2005) Catal Lett 105:259
Zhaoqiong L, Anderson JA (2004) J Catal 224:18
Frola F, Prinetto F, Ghiotti G, Castoldi L, Nova I, Lietti L, Forzatti P (2007) Catal Today 126:81
Nova I, Lietti L, Castoldi L, Tronconi E, Forzatti P (2006) J Catal 239:244
Acknowledgments
The authors would like to thank Natural Sciences and Engineering Research Council of Canada Discovery Grant Program and Kuwait University for financial support and Johnson Matthey for the sample provided.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
AL-Harbi, M., Epling, W.S. Investigating the Effect of NO Versus NO2 on the Performance of a Model NO X Storage/Reduction Catalyst. Catal Lett 130, 121–129 (2009). https://doi.org/10.1007/s10562-009-9912-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-009-9912-3