Abstract
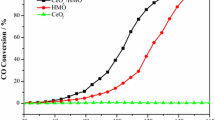
The addition of gold, by deposition precipitation, to a mixed copper manganese oxide catalyst (Hopcalite) has been studied for ambient temperature carbon monoxide oxidation. The deposition of gold on the catalyst surface enhanced the activity of the Hopcalite. The catalyst containing 1 wt% gold was the most active and showed higher activity than Hopcalite containing 0.5 and 2 wt% gold. It is expected that the introduction of gold will introduce new active sites to the Hopcalite that are associated with the gold nanoparticles. However, gold addition also increased the reducibility of the catalyst significantly compared to unmodified Hopcalite, and the most easily reduced catalyst was the most active, indicating that the lability of lattice oxygen was an important factor influencing activity.
Graphical Abstract
The activity of a copper-manganese oxide catalyst for ambient temperature CO oxidation is significantly promoted by the addition of gold using deposition precipitation.






Similar content being viewed by others
References
Jones HA, Taylor HS (1923) J Phys Chem 27:623
Hutchings GJ, Mirzaei AA, Joyner RW, Siddiqui MRH, Taylor SH (1996) Catal Lett 42:21
Hutchings GJ, Mirzaei AA, Joyner RW, Siddiqui MRH, Taylor SH (1998) Appl Catal A 166:143
Mirzaei AA, Shaterian HR, Joyner RW, Stockenhuber M, Taylor SH, Hutchings GJ (2003) Catal Commun 4:17
Lamb, Bray WC, Fraser CW (1920) J Ind Chem Eng 12:213
Jones C, Taylor SH, Burrows A, Crudace MJ, Kiely CJ. Hutchings GJ (2008) Chem Commun 1707
Haruta M, Yamada N, Kobayashi T, Iijima S (1989) J Catal 115:301
Haruta M (1997) J Catal 36:153
Solsona B, Hutchings GJ, Garcia T, Taylor SH (2004) New J Chem 28:708
Spassova, Kristova M, Panayotov D, Mehandjiev D (1999) J Catal 185:43
Hodge NA, Kiely CJ, Whyman R, Siddiqui MRH, Hutchings GJ, Pankhurst QA, Wagner FE, Rajaram RR, Golunski SE (2002) Catal Today 72:133
Fierro G, Lojacono M, Inversi M, Moretti G, Porta P, Lavecchia P (1992) New Front Catal B 75:1847–1850
Oswald HR, Feitknecht W, Wampetich MJ (1965) Nature 207:72
Rogers TH, Piggot CS, Bahlke WH, Jennings JM (1921) J Am Chem Soc 43:1973
Mars P, van Krevelen DW (1954) Chem Eng Sci 3:41
Schwab GM, Kanungo SB (1977) Z Physik Chem N Folge 107:109
Acknowledgements
We would like to thank Molecular Products and the EPSRC for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cole, K.J., Carley, A.F., Crudace, M.J. et al. Copper Manganese Oxide Catalysts Modified by Gold Deposition: The Influence on Activity for Ambient Temperature Carbon Monoxide Oxidation. Catal Lett 138, 143–147 (2010). https://doi.org/10.1007/s10562-010-0392-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-010-0392-2