Abstract
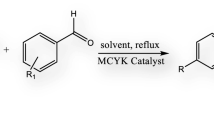
A series of dihydropyrimidin-2(1H)-one (DHPM) belongs to one of the important class of therapeutic and pharmacological active compound, were synthesized through the multicomponent reactions (MCRs) of aldehydes, ethyl acetoacetate and urea, followed by the heterogeneous catalyzed Biginelli reaction. In the present endeavour, medium (ZSM-5) and large pore zeolites (Y, BEA and MOR) as well as dealuminated zeolites BEA, were studied as catalysts. An excellent activity for DHPMs synthesis is achieved by optimizing accessibility of the reactants to the active sites and the surface polarity of zeolite catalysts. Moreover, the mechanism of Biginelli reaction was studied by means of GAUSSVIEW energy calculations of adsorbed acylimine intermediate on zeolite by using the density functional method (DFT).
Graphical Abstract
Zeolite assisted heterogeneous Biginelli reaction







Similar content being viewed by others
References
Kappe CO (1993) Tetrahedron 49:6963
Patil AD, Kumar NV, Kokke WC, Bean MF, Freyer AJ, Brosse CD, Mai S, Truneh A, Faulkner DJ, Carte B, Breen AL, Hertzberg RP, Johnson RK, Westley JW, Potts BCM (1995) J Org Chem 60:1188
Biginelli P (1893) Gazz Chim Ital 23:416
Folkers K, Harwood HJ, Johnson TB (1932) J Am Chem Soc 54:3751
Hu EH, Silder DR, Dolling UHJ (1998) J Org Chem 63:3457
Adapa SR, Alam MM, Varala R (2003) Synlett 1:70
Paraskar AS, Dewkar GK, Sudailal A (2003) Tetrahedron Lett 44:3308
Bussolar JC, McDonnell PA (2000) J Org Chem 65:6779
Khabazzadeh H, Saidi K, Sheibani H (2008) Bioorg Med Chem Lett 18:280
Chen X, Peng Y (2008) Catal Lett 122:313
Yadav JS, Reddy BVS, Reddy KB, Raj KS, Prasad AR (2001) J Chem Soc Perkin Trans 1:1941
Fazaeli R, Tangestaninejad S, Aliyan H, Moghadam M (2006) Appl Catal A Gen 309(1):51
Rafiee E, Shahbazi F (2006) J Mol Catal A Chem 250(1–2):61
Rafiee E, Jafari H (2006) Bioorg Med Chem Lett 16(9):2466
Salehi P, Dabiri M, Zolfigol MA, Bodaghi Fard MA (2003) Tetrahedron Lett 44(14):2891
Shaabani A, Bazgir A, Teimouri F (2003) Tetrahedron Lett 44(4):859
Radha Rani V, Srinivas N, Radhakishan M, Kulkarni SJ, Raghavan KV (2001) Green Chem 3:306
Hegedus A, Hell Z, Vigh I (2006) Synth Commun 36:136
Tajbakhsh M, Mohajerani B, Heravi MM, Ahmadi AN (2005) J Mol Catal A Chem 236(1–2):219
Wagholikar SG, Mayadevi S, Jacob NE, Sivasanker S (2006) Microporous Mesoporous Mater 95:16
Srivastava A, Singh RM (2005) Ind J Chem B 44B:1868
Climent MJ, Corma A, Velty A (2004) Appl Catal A 263:155
Barthomeuf D (1987) Mater Chem Phys 17:49
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.02. Gaussian, Inc., Wallingford
Fernandez AB, Boronat M, Blasco T, Corma A (2005) Angew Chem Int Ed 44:2373
Fernandez AB, Lezcano-Gonzalez I, Boronat M, Blasco T, Corma A (2009) Phys Chem Chem Phys 11:5141
Acknowledgment
The authors are thankful to the Director, SVNIT, Surat, for providing research and financial assistance. The authors would also like to thank Sud-Chemie India Pvt. Ltd., India, for characterization and gift of samples of zeolites.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mistry, S.R., Joshi, R.S., Sahoo, S.K. et al. Synthesis of Dihydropyrimidinones Using Large Pore Zeolites. Catal Lett 141, 1541–1547 (2011). https://doi.org/10.1007/s10562-011-0639-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-011-0639-6