Abstract
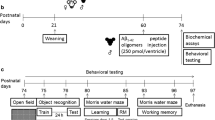
Atrazine (ATR), a widely used herbicide, has been previously shown to damage spatial memory capability and the hippocampus of male rats during the development. It has also been indicated that physical exercise can improve learning and memory in both humans and animals, as a neuroprotective method. Our aim here was to investigate the effect of maternal ATR exposure during gestation and lactation on spatial learning and memory function and hippocampal morphology in offspring and to further evaluate the neuroprotective effect of swimming training and identify possible related learning and memory signaling pathways. Using Sprague-Dawley rats, we examined behavioral and molecular biology effects associated with maternal ATR exposure, as well as the effects of 8 or 28 days swimming training. Maternal exposure to ATR was found to impair spatial learning and memory by behavioral test, damage the hippocampal morphology, and reduce related genes and proteins expression of learning and memory in the hippocampus. The extended, 28 days, period of swimming training produced a greater amelioration of the adverse effects of ATR exposure than the shorter, 8 days, training period. Our results suggest that maternal ATR exposure may damage the spatial learning and memory of offspring male rats via PSD95/NR2B signaling pathway. The negative effect of ATR could be at least partially reversed by swimming training, pointing to a potential neuroprotective role of physical exercise in nervous system diseases accompanying by learning and memory deficit.







Similar content being viewed by others
Abbreviations
- ATR:
-
Atrazine
- IEG:
-
Immediate-early gene
- JNK:
-
c-Jun N-terminal kinase
- NMDA:
-
N-methyl-d-aspartic acid
- NR2B:
-
NMDA receptor subunit 2B
- PI3 K:
-
Phosphatidylinositol 3-kinase
- p-JNK:
-
Phosphorylated c-Jun N-terminal kinase
- PSD95:
-
Postsynaptic density protein-95
References
An TD, Zagaar MA, Levine AT, Salim S, Eriksen J, Alkadhi KA (2013) Treadmill exercise prevents learning and memory impairment in Alzheimer’s disease-like pathology. Curr Alzheimer Res 10:507–515
Biernaskie J, Corbett D (2001) Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci 21:5272–5280
Bustos FJ, Varelanallar L, Campos M, Henriquez B, Phillips M, Opazo C, Aguayo LG, Montecino M, Constantinepaton M, Inestrosa NC (2014) PSD95 suppresses dendritic arbor development in mature hippocampal neurons by occluding the clustering of NR2B-NMDA receptors. PLoS ONE 9:e94037
Chen NN, Luo DJ, Yao XQ, Yu C, Wang Y, Wang Q, Wang JZ, Liu GP (2012) Pesticides induce spatial memory deficits with synaptic impairments and an imbalanced tau phosphorylation in rats. J Alzheimers Dis 30:585–594
Choi J, Park P, Baek G, Sim S, Kang SJ, Lee Y, Ahn S, Lim C, Lee Y, Collingridge GL (2014) Effects of PI3Kγ overexpression in the hippocampus on synaptic plasticity and spatial learning. Mol Brain 7:78
Coley AA, Gao WJ (2017) PSD95: a synaptic protein implicated in schizophrenia or autism? Prog Neuropsychopharmacol Biol Psychiatry 82:187–194
Counts SE, Alldred MJ, Che S, Ginsberg SD, Mufson EJ (2014) Synaptic gene dysregulation within hippocampal CA1 pyramidal neurons in mild cognitive impairment. Neuropharmacology 79:172–179
Cyna M, Lynch M, Oore JJ, Nagy PM, Aubert I (2016) The benefits of exercise and metabolic interventions for the prevention and early treatment of Alzheimer’s disease. Curr Alzheimer Res 14:47–60
Dao AT, Zagaar MA, Alkadhi KA (2015) Moderate treadmill exercise protects synaptic plasticity of the dentate gyrus and related signaling cascade in a rat model of Alzheimer’s disease. Mol Neurobiol 52:1067–1076
De Ferrari GV, Avila ME, Medina MA, Perez-Palma EP, Bustos BI, Alarcon MA (2014) Wnt/β-catenin signaling in Alzheimer’s disease. CNS Neurol Disord: Drug Targets 13:745–754
Delint-Ramírez I, Salcedo-Tello P, Bermudez-Rattoni F (2008) Spatial memory formation induces recruitment of NMDA receptor and PSD-95 to synaptic lipid rafts. J Neurochem 106:1658–1668
D’Mello R, Marchand F, Pezet S, Mcmahon SB, Dickenson AH (2011) Perturbing PSD-95 interactions with NR2B-subtype receptors attenuates spinal nociceptive plasticity and neuropathic pain. Mol Ther 19:1780–1792
Dong F, Mitchell PD, Davis VM, Recker R (2017) Impact of atrazine prohibition on the sustainability of weed management in Wisconsin maize production. Pest Manag Sci 73:425–434
Eldridge JC, Tennant MK, Wetzel LT, Breckenridge CB, Stevens JT (1994) Factors affecting mammary tumor incidence in chlorotriazine-treated female rats: hormonal properties, dosage, and animal strain. Environ Health Perspect 102(Suppl 11):29–36
Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci 108:3017–3022
Fang G, Zhao J, Zhang L, Li P, Liang LI, Yang X, Tao YU (2017) Effects of aerobic exercise on Wnt/β-catenin pathway in the cerebral cortex and hippocampus of rats. Chin J Sports Med 36:765–772
Farías GG, Alfaro IE, Cerpa W, Grabowski CP, Godoy JA, Bonansco C, Inestrosa NC (2009) Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem 284:15857
Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B (2009) Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry 33:70–75
Folke J, Pakkenberg B, Brudek T (2018) Impaired Wnt signaling in the prefrontal cortex of Alzheimer’s disease. Mol Neurobiol 56:873
Fouquet M, Desgranges B, La JR, Rivière D, Mangin JF, Landeau B, Mézenge F, Pélerin A, De LSV, Viader F (2012) Role of hippocampal CA1 atrophy in memory encoding deficits in amnestic mild cognitive impairment. Neuroimage 59:3309–3315
Gallo FT, Katche C, Morici JF, Medina JH, Weisstaub NV (2018) Immediate early genes, memory and psychiatric disorders: focus on c-Fos, Egr1 and Arc. Front Behav Neurosci 12:79
Hillman CH, Erickson KI, Kramer AF (2008) Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci 9:58–65
Jae-Ick K, Hye-Ryeon L, Su-eon S, Jinhee B, Nam-Kyung Y, Jun-Hyeok C, Hyoung-Gon K, Yong-Seok L, Soo-Won P, Chuljung K (2011) PI3 Kγ is required for NMDA receptor-dependent long-term depression and behavioral flexibility. Nat Neurosci 14:1447–1454
Kornau HC, Schenker LT, Kennedy MB, Seeburg PH (1995) Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269:1737–1740
Kramer K, Dijkstra H, Bast A (1993) Control of physical exercise of rats in a swimming basin. Physiol Behav 53:271–276
Li XU, Ling LI, Yan PS, Hospital XJ (2000) Change of histo-morphology on the rats’ cerebral infarction after rehabilitative training. Mod Rehabilit 4:678–679
Li C, Dong S, Wang H, Hu Y (2011) Microarray analysis of gene expression changes in the brains of NR2B-induced memory-enhanced mice. Neuroscience 197:121–131
Li B, He X, Sun Y, Li B (2016) Developmental exposure to paraquat and maneb can impair cognition, learning and memory in Sprague-Dawley rats. Mol BioSyst 12:3088–3097
Li B, Jia S, Yue T, Yang L, Huang C, Verkhratsky A, Peng L (2017) Biphasic regulation of Caveolin-1 gene expression by fluoxetine in astrocytes: opposite effects of PI3K/AKT and MAPK/ERK signaling pathways on c-fos. Front Cell Neurosci 11:335
Li J, Li X, Bi H, Ma K, Li B (2018) Developmental exposure to atrazine impairs spatial memory and downregulates the hippocampal D1 dopamine receptor and cAMP-dependent signaling pathway in rats. Int J Mol Sci 19:2241
Liu PZ, Nusslock R (2018) Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci 12:52
Liu Z, Fu Z, Jin Y (2017) Immunotoxic effects of atrazine and its main metabolites at environmental relevant concentrations on larval zebrafish (Danio rerio). Chemosphere 166:212–220
Millan MJ, Yves A, Martin B, Bullmore ET, Carter CS, Clayton NS, Richard C, Sabrina D, Bill D, Derubeis RJ (2012) Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov 11:141–168
Mostafalou S, Abdollahi M (2017) Pesticides: an update of human exposure and toxicity. Arch Toxicol 91:549–599
Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B (2017) Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med 52:154–160
Oertel-Knöchel V, Mehler P, Thiel C, Steinbrecher K, Malchow B, Tesky V, Ademmer K, Prvulovic D, Banzer W, Zopf Y, Schmitt A, Hänsel F (2014) Effects of aerobic exercise on cognitive performance and individual psychopathology in depressive and schizophrenia patients. Eur Arch Psychiatry Clin Neurosci 264:589–604
Patki G, Li L, Allam F, Solanki N, Dao AT, Alkadhi K, Salim S (2014) Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiol Behav 130:47–53
Prakash RS, Voss MW, Erickson KI, Kramer AF (2015) Physical activity and cognitive vitality. Annu Rev Psychol 66:769–797
Rendeiro C, Rhodes JS (2018) A new perspective of the hippocampus in the origin of exercise–brain interactions. Brain Struct Funct 223:2527–2545
Riise J, Plath N, Pakkenberg B, Parachikova A (2015) Aberrant Wnt signaling pathway in medial temporal lobe structures of Alzheimer’s disease. J Neural Transmission 122:1303–1318
Rimayi C, Odusanya D, Weiss JM, de Boer J, Chimuka L (2018) Seasonal variation of chloro-s-triazines in the Hartbeespoort Dam catchment, South Africa. Sci Total Environ 613–614:472–482
Rustemeyer J, Krajacic A, Dicke U (2010) Histomorphological and functional impacts of postoperative motor training in rats after allograft sciatic nerve transplantation under low-dose FK 506. Muscle Nerve 39:480–488
Sai L, Dong Z, Li L, Guo Q, Jia Q, Xie L, Bo C, Liu Y, Qu B, Li X (2016) Gene expression profiles in testis of developing male Xenopus laevis damaged by chronic exposure of atrazine. Chemosphere 159:145–152
Shaw G (2011) New evidence for association of pesticides with parkinson disease. Neurol Today 11(1):16–21
Stranahan AM, Lee K, Becker KG, Zhang Y, Maudsley S, Martin B, Cutler RG, Mattson MP (2010) Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol Aging 31:1937–1949
Sun QJ, Duan RS, Wang AH, Shang W, Zhang T, Zhang XQ, Chi ZF (2009) Alterations of NR2B and PSD-95 expression in hippocampus of kainic acid-exposed rats with behavioural deficits. Behav Brain Res 201:292–299
Sun Y, Li YS, Yang JW, Yu J, Wu YP, Li BX (2014) Exposure to atrazine during gestation and lactation periods: toxicity effects on dopaminergic neurons in offspring by downregulation of Nurr1 and VMAT2. Int J Mol Sci 15:2811
Tanabe Y, Hashimoto M, Sugioka K, Maruyama M, Fujii Y, Hagiwara R, Hara T, Hossain SM, Shido O (2010) Improvement of spatial cognition with dietary docosahexaenoic acid is associated with an increase in Fos expression in rat CA1 hippocampus. Clin Exp Pharmacol Physiol 31:700–703
Van M-FG, Hoet P, Vilain F, Lison D (2012) Occupational exposure to pesticides and Parkinson’s disease: a systematic review and meta-analysis of cohort studies. Environ Int 46:30
Walters JL, Lansdell TA, Lookingland KJ, Baker LE (2015) The effects of gestational and chronic atrazine exposure on motor behaviors and striatal dopamine in male Sprague-Dawley rats. Toxicol Appl Pharmacol 289:185–192
Weber GJ, Sepúlveda MS, Peterson SM, Lewis SS, Freeman JL (2013) Transcriptome alterations following developmental atrazine exposure in zebrafish are associated with disruption of neuroendocrine and reproductive system function, cell cycle, and carcinogenesis. Toxicol Sci 132:458–466
Wirbisky SE, Weber GJ, Sepúlveda MS, Lin TL, Jannasch AS, Freeman JL (2016) An embryonic atrazine exposure results in reproductive dysfunction in adult zebrafish and morphological alterations in their offspring. Sci Rep 6:21337
Xing H, Li S, Wang X, Gao X, Xu S, Wang X (2013) Effects of atrazine and chlorpyrifos on the mRNA levels of HSP70 and HSC70 in the liver, brain, kidney and gill of common carp (Cyprinus carpio L.). Chemosphere 90:910–916
Ying Z, Bingaman W, Najm IM (2010) Increased Numbers of Coassembled PSD-95 to NMDA-receptor subunits NR2B and NR1 in human epileptic cortical dysplasia. Epilepsia 45:314–321
Zaya RM, Amini Z, Whitaker AS, Kohler SL, Ide CF (2011) Atrazine exposure affects growth, body condition and liver health in Xenopus laevis tadpoles. Aquat Toxicol 104:243
Acknowledgements
We are grateful to Di Huang and Xi Li at the Harbin Medical University for critically revising the manuscript and performing the statistical analysis. There was no specific grant from funding agencies in the public, commercial, or not-for-profit sectors in this research.
Author information
Authors and Affiliations
Contributions
BL, DW, and BL all participated in the study design. DW, BL, and YW conducted the experiments. DW performed the data analysis and the writing of the manuscript. BL read the final manuscript and provided financial support.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, D., Li, B., Wu, Y. et al. The Effects of Maternal Atrazine Exposure and Swimming Training on Spatial Learning Memory and Hippocampal Morphology in Offspring Male Rats via PSD95/NR2B Signaling Pathway. Cell Mol Neurobiol 39, 1003–1015 (2019). https://doi.org/10.1007/s10571-019-00695-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-019-00695-3