Abstract
Background
Hepatic stellate cells (HSC) play a major role in the progression of liver fibrosis.
Aim
The aim of our study was to investigate whether rat HSC cultured on a nanofiber membrane (NM) retain their quiescent phenotype during both short- and long-term culture and whether activated HSC revert to a quiescent form when re-cultured on NM.
Methods
Rat HSC cultured for 1 day on plastic plates (PP) were used as quiescent HSC, while cells cultured for 1 week on PP were considered to be activated HSC. Quiescent or activated HSC were subsequently plated on PP or NM and cultured for an additional 4 days at which time their gene expression, stress fiber development, and growth factor production were determined. For long-term culture, HSC were grown on NM for 20 days and the cells then replated on PP and cultured for another 10 days.
Results
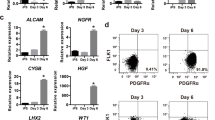
Expression of marker genes for HSC activation, stress fiber development, and growth factor production were significantly lower in both quiescent and activated HSC cultured on NM than in those cultured on PP. After long-term culture on NM, activation marker gene expression and stress fiber development were still significantly lower in HSC than in PP, and HSC still retained the ability to activate when replated onto PP.
Conclusions
HSC cultured on NM retained quiescent characteristics after both short- and long-term culture while activated HSC reverted toward a quiescent state when cultured on NM. Cultures of HSC grown on NM are a useful in vitro model to investigate the mechanisms of activation and deactivation.





Similar content being viewed by others
References
Friedman SL. Liver fibrosis -from bench to bedside. J Hepatol. 2003;3838–53.
Tsukada S, Parsons CJ, Rippe RA. Mechanisms of liver fibrosis. Clini Chim Acta. 2006;64:33–60.
Eng FJ, Friedman SL, Fibrogenesis I. New insights into hepatic stellate cell activation: the simple becomes complex. Am J Physiol Gastrointest Lver Physiol. 2000;279:G7–G11.
Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250.
de Leeuw AM, McCarthy SP, Geerts A, Knook DL. Purified rat liver fat-storing cells in culture divide and contain collagen. Hepatology. 1984;4:392–403.
Knittel T, Kobold D, Dudas J, Saile B, Ramadori G. Role of the Ets-1 transcription factor during activation of rat hepatic stellate cells in culture. Am J Pathol. 1999;155:1841–1848.
Liu C, Gaça MD, Swenson ES, Vellucci VF, Reiss M, Wells RG. Smads 2 and 3 are differentially activated by transforming growth factor-beta (TGF-beta) in quiescent and activated hepatic stellate cells. Constitutive nuclear localization of Smads in activated cells is TGF-beta-independent. J Biol Chem. 2003;278:11721–11728.
Li YL, Sato M, Kojima N, Miura M, Senoo H. Regulatory role of extracellular matrix components in expression of matrix metalloproteinases in cultured hepatic stellate cells. Cell Struct Funct. 1999;24:255–261.
Sohara N, Znoyko I, Levy MT, Trojanowska M, Reuben A. Reversal of activation of human myofibroblast-like cells by culture on a basement membrane-like substrate. J Hepatol. 2002;37:214–221.
Gaça MD, Zhou X, Issa R, Kiriella K, Iredale JP, Benyon RC. Basement membrane-like matrix inhibits proliferation and collagen synthesis by activated rat hepatic stellate cells: evidence for matrix-dependent deactivation of stellate cells. Matrix Biol. 2003;22:229–239.
Znoyko I, Trojanowska M, Reuben A. Collagen binding α2β1 and α1β1 integrins play contrasting roles in regulation of Ets-1 expression in human liver myofibroblasts. Mol Cell Biochem. 2006;282:89–99.
Shimada H, Ochi T, Imasato A, et al. Gene expression profiling and functional assays of activated hepatic stellate cells suggest that myocardin has a role in activation. Liver Int. 2010;30:42–54.
Friedman SL, Roll FJ, Boyles J, Arenson DM, Bissell DM. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem. 1989;264:10756–10762.
Ondarcuhu T, Joachim C. Drawing a single nanofibre over hundreds of microns. Europhys Lett. 1998;42:215–220.
Whitesides GM, Grzybowski BA. Dynamic aggregation of chiral spinners. Science. 2002;296:718–721.
Hosseinkhani H, Hosseinkhani M, Tian F, Kobayashi H, Tabata Y. Ectopic bone formation in collagen sponge self-assembled peptide-amphiphile nanofibers hybrid scaffold in a perfusion culture bioreactor. Biomaterials. 2006;27:5089–5098.
Hosseinkhani H, Hosseinkhani M, Tian F, Kobayashi H, Tabata Y. Osteogenic differentiation of mesenchymal stem cells in self-assembled peptide-amphiphile nanofibers. Biomaterials. 2006;27:4079–4086.
Shih YR, Chen CN, Tsai SW, Wang YJ, Lee OK. Growth of mesenchymal stem cells on electrospun type I collagen nanofibers. Stem Cells. 2006;24:2391–2397.
Mohammadi Y, Soleimani M, Fallahi-Sichani M, et al. Nanofibrous poly (epsilon-caprolactone)/poly (vinylalcohol)/chitosan hybrid scaffolds for bone tissue engineering using mesenchymal stem cells. Int J Artif Organs. 2007;30:204–211.
Xin X, Hussain M, Mao JJ. Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials. 2007;28:316–325.
Fong H, Reneker DH. Electrospinning and formation of nanofibers. In: Salem DR, ed. Structure Formation in Polymeric Fibers. Munich: Hanser; 2001:225–246.
Teo WE, He W, Ramakrishna S. Electrospun scaffold tailored for tissue specific extracellular matrix. Biotechnol J. 2006;1:918–929.
Baker BM, Handorf AM, Ionescu LC, Li WJ, Mauck RL. New directions in nanofibrous scaffolds for soft tissue engineering and regeneration. Exp Rev Med Devices. 2009;6:515–532.
Lim SH, Mao HQ. Electrospun scaffolds for stem cell engineering. Adv Drug Deliv Rev. 2009;61:1084–1096.
Kojima N, Hori M, Murata T, Morizane Y, Ozaki H. Different profiles of Ca2+ responses to endothelin-1 and PDGF in liver myofibroblasts during the process of cell differentiation. Br J Pharmacol. 2007;151:816–827.
Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc. 2009;120:361–368.
Sato M, Suzuki S, Senoo H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003;28:105–112.
Olsen AL, Bloomer SA, Chan EP, et al. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 2011;301:G110–G118.
Pinzani M, Marra F, Carloni V. Signal transduction in hepatic stellate cells. Liver. 1998;18:2–13.
Ankoma-Sey V, Matli M, Chang KB, et al. Coordinated induction of VEGF receptors in mesenchymal cell types during rat hepatic wound healing. Oncogene. 1998;17:115–121.
Madsen CD, Sidenius N. The interaction between urokinase receptor and vitronectin in cell adhesion and signalling. Eur J Cell Biol. 2008;87:617–629.
Préaux AM, Mallat A, Nhieu JT, D’Ortho MP, Hembry RM, Mavier P. Matrix metalloproteinase-2 activation in human hepatic fibrosis regulation by cell-matrix interactions. Hepatology. 1999;30:944–950.
Wang DR, Sato M, Sato T, Kojima N, Higashi N, Senoo H. Regulation of matrix metallo-proteinase expression by extracellular matrix components in cultured hepatic stellate cells. Comp Hepatol. 2004;3:S20.
Pinzani M, Milani S, De Franco R, et al. Endothelin 1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology. 1996;110:534–548.
Rockey DC, Fouassier L, Chung JJ, et al. Cellular localization of endothelin-1 and increased production in liver injury in the rat: potential for autocrine and paracrine effects on stellate cells. Hepatology. 1998;27:472–480.
Mallat A, Préaux AM, Serradeil-Le Gal C, et al. Growth inhibitory properties of endothelin-1 in activated human hepatic stellate cells: a cyclic adenosine monophosphate-mediated pathway. Inhibition of both extracellular signal-regulated kinase and c-Jun kinase and upregulation of endothelin B receptors. J Clin Invest. 1996;98:2771–2778.
Serini G, Gabbiana G. Modulation of alpha-smooth muscle actin expression in fibroblasts by transforming growth factor-beta isoforms: an in vivo and in vitro study. Wound Repair Regen. 1996;4:278–287.
Batra V, Musani AI, Hastie AT, et al. Bronchoalveolar lavage fluid concentrations of transforming growth factor (TGF)-beta1, TGF-beta2, interleukin (IL)-4 and IL-13 after segmental allergen challenge and their effects on alpha-smooth muscle actin and collagen III synthesis by primary human lung fibroblasts. Clin Exp Allergy. 2004;34:437–444.
Serpero L, Petecchia L, Sabatini F, et al. The effect of transforming growth factor (TGF)-beta1 and (TGF)-beta2 on nasal polyp fibroblast activities involved upper airway remodeling: modulation by fluticasone propionate. Immunol Lett. 2006;105:61–67.
Shimada H, Staten NR, Rajagopalan LE. TGF-β1 mediated activation of Rho kinase induces TGF-β2 and endothelin-1 expression in human hepatic stellate cells. J Hepatol. 2011;54:521–528.
Wettschureck N, Offermanns S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J Mol Med (Berl). 2002;80:629–638.
Sohail MA, Hashmi AZ, Hakim W, et al. Adenosine induces loss of actin stress fibers and inhibits contraction in hepatic stellate cells via Rho inhibition. Hepatology. 2009;49:185–194.
Murata T, Arii S, Nakamura T, et al. Inhibitory effect of Y-27632, a ROCK inhibitor, on progression of rat liver fibrosis in association with inactivation of hepatic stellate cells. J Hepatol. 2001;35:474–481.
Fukushima M, Nakamuta M, Kohjima M, et al. Fasudil hydrochloride hydrate, a Rho-kinase (ROCK) inhibitor, suppresses collagen production and enhances collagenase activity in hepatic stellate cells. Liver Int. 2005;25:829–838.
Shafiei MS, Rockey DC. The function of integrin-linked kinase in normal and activated stellate cells: implications for fibrogenesis in wound healing. Lab Invest. 2012;92:305–316
Wells RG, Kruglov E, Dranoff JA. Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett. 2004;559:107–110.
Acknowledgments
This work is supported by Pfizer Inc.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eda, H., Kulig, K.M., Steiner, T.A. et al. A Nanofiber Membrane Maintains the Quiescent Phenotype of Hepatic Stellate Cells. Dig Dis Sci 57, 1152–1162 (2012). https://doi.org/10.1007/s10620-012-2084-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2084-9