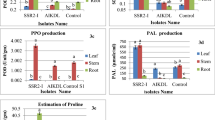
Abstract
A study of the effect of bioagents and dipotassium phosphate (DPP) and their combination on early blight disease reduction under greenhouse conditions was conducted. Native bacterial isolates as bio-control agents exhibited control against early blight. Five bacterial isolates were tested against the pathogen. All isolates exhibited significant antagonistic activity against Alternariasolani, isolate “bact-03” showed significant in vitro inhibition (42.6%) and later was identified as Bacillus amyloliquefaciensby 16S rDNA gene analysis. Tests conducted on dipotassium phosphate (DPP) at different concentrations (10 mM, 25 mM, and 50 mM) showed mycelial growth inhibition 14.2%, 27.4%, and 54.8%, respectively. In vitro synergetic study on seed germination showed that the combination of DPP and B. amyloliquefaciens antagonized the pathogen. Vigor index was also significant in the combination (343.0) as well as DPP (299.0), and bioagent (426.6) compared to control (170.0). In the in vivo application of B. amyloliquefaciens, DPP combination showed significant disease reduction. However, disease severity on the plants treated with DPP was 35%, and the plants treated with B. amyloliquefaciens was 30% while in combination of both showed the disease severity up to 42% that was significantly lower than control (82%). Application of these bioagents and DPP also sustained the plant weight by promoting the growth and development of plant. The results of this study indicate that naturally existing bioagents along with the slats of potassium phosphate may provide promising control of early blight disease.. Due to their antagonistism, bacterial strains with the combination of salts can be used as bio-pesticides. Their applicationsimproves seed health and crop yield, but signaling relationship in pathogens, plants, and soil still needs to divulge to promote BCAs as encouraging bio-pesticides for the future.






Similar content being viewed by others
References
Abdel-Kader, M. M., El-Mougy, N. S., & Lashin, S. M. (2013). Biological and chemical resistance inducers approaches for controlling foliar diseases of some vegetables under protected cultivation system. Journal of Plant Pathology and Microbiology, 4, 1–9.
Abo-Elyousr, K.A.M., Hussein, M.A.M., Allam, A.D.A., Hassan, M.H.A. (2008). Enhanced onion resistance against stemphylium leaf blight disease, caused by Stemphyliumvesicarium, by di-potassium phosphate and benzothiadiazole treatments. Plant Pathology Journal, 24(2), 171–177.
Abo-Elyousr, K. A. M., & Hadeel, M. M. K. (2019). Biological control of the tomato wilt caused by Clavibactermichiganensis subsp michiganensis using formulated plant growth-promoting bacteria. Egyptian Journal of Biological Pest Control., 29, 54.
Abo-Elyousr, K. A. M., & El-Hendawy H.-H. (2008). Integration of Pseudomonas fluorescens and acibenzolar-S-methyl to control bacterial spot disease of tomato. Crop Protection, 27, 1118–1124.
Abo-Elyousr, K. A. M., Najeeb, M. A., Ahmed, W. M. A., Sergio, R. R., & Khamis, Y. (2020). Plant extract treatments induce resistance to bacterial spot by tomato plants for a sustainable system. Horticulture, 6(36), 1–12.
Ajilogba, C. F., Babalola, O. O., & Ahmad, F. (2013). Antagonistic effects of Bacillus species in biocontrol of tomato Fusarium wilt. Studies on Ethno-Medicine., 7(3), 205–216.
Alexis, G., Dinka, M., & Mauricio, G. (2020). Isolation and identification of soil bacteria from extreme environments of chile and their plant beneficial characteristics. Microorganisms., 8, 1213.
Arguelles-Arias, A., Ongena, M., Halimi, B., Lara, Y., Brans, A., Joris, B., et al. (2009). Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microbial Cell Factories., 8, 63. https://doi.org/10.1186/1475-2859-8-63
Ayşe, A., & Katirciolu, Y. (2008). Determination of pathogens causing damping-off and their pathogenicity in tomato seedbeds in Ankara (Ayaş, Beypazarı and Nallıhan districts) province. Plant Protection Bulletin., 48(2), 49–59.
Bereika, F. F. M., Nashwa, M. A. S., Saad, A. M. A., Kamal, A.-E., Mohamed, H., & Yasser, S. M. (2020). Approving the biocontrol strategy of potato wilt caused by Ralstonia solanacearum on field scale using Enterobacter cloacae PS14 and Trichoderma asperellum T34. Egyptian Journal of Biological Pest Control, 30, 61.
Bharath, B. G., Lokesh, S., & Shetty, H. S. (2005). Effects of fungicides and bioagents on seed Mycoflora, growth and yield of watermelon. Integrative Biosciences., 9(2), 75–78. https://doi.org/10.1080/17386357.2005.9647254
Bin, L., Shida, J., Huifang, Z., Yucheng, W., & Zhihua, L. (2020). Isolation of Trichoderma in the rhizosphere soil of Syringa oblata from Harbin and their biocontrol and growth promotion function. Microbiol Research., 235, 126445.
Casals, C., Teixido, N., Vinas, I., Cambray, J., & Usall, J. (2010). Control of Monilinia spp. on stone fruit by curing treatments. Part II: The effect of host and Monilinia spp. variables on curing efficacy. Postharvest Biology and Technology., 56, 26–30.
Chen, Y., Yan, F., Chai, Y., Liu, H., Kolter, R., Losick, R., et al. (2013). Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environmental Microbiology., 15, 848–864.
Diener, A. C., & Ausubel, F. M. (2005). Resistance to Fusariumoxysporum 1, a dominant Arabidopsis disease-resistance gene, is not race specific. Genetics, 171, 305–321.
Dipak, T. N, Anil, P. G., &Lalan, S. (2013) Morphological and cultural characterization of Alternaria alternata (Fr) Keissler blight of gerbera (Gerbera jamesonii H Bolus ex JD Hook) Journal of Applied and Natural Science. 5 (1): 171–178.
Ehret, D. L., Menzies, J. G., Bogdanoff, C., Ukthede, R. S., & Frey, B. (2002). Foliar applications of fertilizer salts inhibit powdery mildew on tomato. Canadian Journal of Plant Pathology., 24, 437–444.
El-Mougy, N. S., & Abdel-Kader, M. M. (2013). Effect of commercial cyanobacteria products on the growth and antagonistic ability of some bioagents under laboratory conditions. Journal of Pathogens., 2013(838329), 11.
Esra, C., & Elif, T. (2019). Biological control of Alternariasolani in tomato. Fresenius Environmental Bulletin., 28(10), 7092–7100.
Felsenstein, J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution, 39, 783–791.
Freitas, M. A., Medeiros, F. H. V., Melo, I. S., Pereira, P. F., Peñaflor, M. F. G. V., Bento, J. M. S., et al. (2019). Stem inoculation with bacterial strains Bacillus amyloliquefaciens (GB03) and Microbacterium imperiale (MAIIF2a) mitigates fusarium root rot in cassava. Phytoparasitica, 47, 135–142.
Gerhardt, P., Murray, R., Costilow, R., Nester, E. W., Wood, W. A., Krieg, N., & Phillips, G. B. (1981). Manual of Methods for General Bacteriology., 34(9), 1069.
Grigolli, J. F. J., Kubota, M. M., Alves, D. P., Rodrigues, G. B., Cardoso, C. R., Henriques da Silva, D. J., & Mizubuti, E. S. G. (2011). Characterization of tomato accessions for resistance to early blight. Crop Breeding and Applied Biotechnology., 11, 174–180.
Hanqin, X., Yongtao, L., Yanfei, C., Yu, C., & Yan, W. (2015). Isolation of Bacillus amyloliquefaciens JK6 and identification of its lipopeptides surfactin for suppressing tomato bacterial wilt. RSC Advances., 5, 82042–82049. https://doi.org/10.1039/C5RA13142A
Huang, J., Wei, Z., Tan, S., Mei, X., Shen, Q., & Xu, Y. (2014). Suppression of bacterial wilt of tomato by bioorganic fertilizer made from the antibacterial compound producing strain bacillus amyloliquefaciens HR62. Journal of Agriculture and Food Chemistry, 62, 10708–10716.
Imran, M., Esmat, F. A., Kamal, A. M. A., Nashwa, M. A. S., Muhammad, M. M. K., & Muhammad, W. Y. (2021). Characterization and sensitivity of Botrytis cinerea to Benzimidazole and SDHI fungicides and illustration of resistance profile. Australasian Plant Pathology. https://doi.org/10.1007/s13313-021-00803-2
Karuppiah, V., Sun, J., Li, T., Vallikkannu, M., & Chen, J. (2019). Co-cultivation of Trichoderma asperellum GDFS1009 and Bacillus amyloliquefaciens 1841 Causes Differential Gene Expression and Improvement in the Wheat Growth and Biocontrol Activity. Frontiers in Microbiology, 10, 1068.
Khalil, S. A. M., Nehal, S.-M., Nadia, G.-G., & Mokhtar, M.-K. (2020). Field approaches of chemical inducers and bioagents for controlling root diseases incidence of pea (Pisumsativum L.) under field conditions. The Plant Pathology Journal., 19(3), 166–175.
Khan, N., Martínez-Hidalgo, P., Ice, T. A., Maymon, M., Humm, E. A., Nejat, N., Sanders, E. R., Kaplan, D., & Hirsch, A. M. (2018). Antifungal Activity of Bacillus Species against Fusarium and Analysis of the Potential Mechanisms Used in Biocontrol. Frontiers in Microbiology., 9, 2363.
Kim, J., Rohlf, F. J., & Sokal, R. R. (1993). The accuracy of phylogenetic estimation using the neighbor-joining method. Evolution, 2, 471–486.
Kim, P., Bai, H., Bai, D., Chae, H., Chung, S., Kim, Y., et al. (2004). Purification and characterization of a lipopeptide produced by Bacillus thuringiensis CMB26. The Journal of Applied Microbiology., 97, 942–949. https://doi.org/10.1111/j.1365-2672.2004.02356.x
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution., 35, 1547–1549.
Li, J., Yan, Q., Zhao, L. H., Zhang, S. M., Wang, Y. X., & Zhao, X. Y. (2009). Purification and characterization of a novel antifungal protein from Bacillus subtilis strain B29. The Journal of Zhejiang University Science b., 10, 264–272. https://doi.org/10.1631/jzus.B0820341
Li, L., Ma, J., Li, Y., Wang, Z., Gao, T., & Wang, Q. (2012). Screening and partial characterization of Bacillus with potential applications in biocontrol of cucumber Fusarium wilt. Crop Protection., 35, 29–35.
Lin, C., Tsai, C. H., Chen, P. Y., Wu, C. Y., Chang, Y. L., Yang, Y. L., et al. (2018). Biological control of potato common scab by Bacillus amyloliquefaciens Ba01. PLoS ONE, 13(4), e0196520. https://doi.org/10.1371/journal.pone.0196520
Manandhar, H. K., Jorgensen, H. J. L., Mathur, S. B., & Smedegaard-Petersen, V. (1998). Resistance to rice blast induced by ferric chloride, dipotassium hydrogen phosphate and salicylic acid. Crop Protection., 17, 323–329.
Marchesi, J. R., Sato, T., Weightman, A. J., Martin, T. A., Fry, J. C., Hiom, S. J., & Wade, W. G. (1998). Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rDNA. Applied and Environmental Microbiology., 64, 795–799.
Merzoug, A., BeLABid, L., Youcef-BenkAdA, M., Benfreha, F., & Bayaa, B. (2014). Pea Fusarium wilt races in western Algeria. Plant Protection Science., 50, 70–77.
Nashwa, S. M. A., & Abo-Elyousr, K. A. M. (2012). Evaluation of various plant extracts against the early blight disease of tomato plants under greenhouse and field conditions. Plant Protect Sci, 48(2), 75–80.
Nigro, F., Schena, L., Ligorio, A., Pentimone, I., Ippolito, A., & Salerno, M. G. (2006). Control of table grape storage rots by pre-harvest applications of salts. Postharvest Biology and Technology., 42, 142–149.
Orober, M., Siegrist, J., & Buchenauer, H. (2002). Mechanisms of phosphate-induced disease resistance in cucumber. European Journal of Plant Pathology., 108, 345–353.
Pasche, J., Wharam, C., & Gudmestad, N. (2004). Shift in sensitivity of Alternariasolani in response to QoI fungicides. Plant Disease, 88, 181–187. https://doi.org/10.1094/pdis.2004.88.2.181
Peters, R., Drake, K., Gudmestad, N., Pasche, J., & Shinners-Carnelley, T. (2008). First report of reduced sensitivity to a QoI fungicide in isolates of Alternariasolani causing early blight of potato in Canada. Plant Disease, 92, 1707–1707. https://doi.org/10.1094/pdis-92-12-1707b
Pretorius, D., van Rooyen, J., & Clarke, K. G. (2015). Enhanced production of antifungal lipopeptides by Bacillus amyloliquefaciens for biocontrol of postharvest disease. New Biotechnology, 32, 243–252.
Priest, F. G., Goodfellow, M., Shute, L. A., & Berkeley, R. C. W. (1987). Bacillus amyloliquefaciensspnov, nom rev. International Journal of Systematic Bacteriology, 37, 69–71. https://doi.org/10.1099/00207713-37-1-69
Qiu, M., Wu, C., Ren, G., Liang, X., Wang, X., & Huang, J. (2014). Effect of chitosan and its derivatives as antifungal and preservative agents on postharvest green asparagus. Food Chemistry, 155, 105–111.
Reuveni, R., Dor, G., & Reuveni, M. (1998). Local and systemic control of powdery mildew (Leveillulataurica) on pepper plants by foliar spray of mono-potassium phosphate. Crop Protection, 17, 703–709.
Reuveni, R., Dor, G., Raviv, M., Reuveni, M., & Tuzun, S. (2000). Systemic resistance against Sphaerothecafuliginea in cucumber plants exposed to phosphate in hydroponics system and its control by foliar spray of mono potassium phosphate. Crop Protection, 19, 355–361.
Saitou, N., & Nei, M. (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4, 406–425.
Sambrook, J., Russell, D. W., Janssen, K., & Argentine, J. (2001). Molecular Cloning: A Laboratory Manual (3rd ed.). Cold Spring Harbor Laboratory.
Sharma, R., Singh, D., & Singh, R. (2009). Biological control of postharvest diseases of fruits and vegetables by microbial antagonists. A Review in Biological Control, 50, 205–221.
Sharma-Poudyal, D., Paulitz, T. C., Porter, L. D., & du Toit, L. J. (2015). Characterization and pathogenicity of Rhizoctonia and Rhizoctonia-like spp. from pea crops in the Columbia basin of Oregon and Washington. Plant Disease, 99, 604–613.
Shashidar, A., Marc, O., Delphine, D., Edwin, D. P., Kunling, C., Sarosh, B., & Johan, M. (2017). Insights into the molecular basis of biocontrol of Brassica pathogens by Bacillus amyloliquefaciens UCMB5113 lipopeptides. Annals of Botany., 120, 551–562.
Siahmoshteh, F., et al. (2017). Efficacy of Bacillus subtilis and Bacillus amyloliquefaciens in the control of Aspergillus parasiticus growth and aflatoxins production on pistachio. International Journal of Food Microbiology., 254, 47–53.
Siahmoshteh, F., Zohreh, H. E., Davide, S., Masoomeh, S. G., & Mehdi, R. A. (2018). Unraveling the mode of antifungal action of Bacillus subtilis and Bacillus amyloliquefaciens as potential biocontrol agents against aflatoxigenic Aspergillus parasiticus. Food Control, 89, 300–307.
Somnath, K., Shyama, S. M., & Kole, P. C. (2015). In vitro efficacy of bio-control agents and botanicals on the growth inhibition of Alternariasolani causing early leaf blight of tomato. International Journal of Bio-Resource, Environment and Agricultural Sciences., 1(3), 114–118.
Steel, R.G.D., Torrie, J.H., & Dickey, D.A. (1996) Principles and Procedures of Statistics: A Biometric Approach, 3rd ed, McGraw Hill Book Co. Inc.: New York, NY, USA
Stein, T. (2005). Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Molecular Microbiology., 56, 845–857.
Tamura, K., Nei, M., & Kumar, S. (2004). Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences (USA), 101, 11030–11035.
Tan, S., Gu, Y., Yang, C., Dong, Y., Mei, X., Shen, Q., et al. (2016). Bacillus amyloliquefaciens T-5 may prevent Ralstonia solanacearum infection through competitive exclusion. Biology and Fertility of Soils, 52, 341–351.
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., & Higgins, D. G. (1997). The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research., 25, 4876–4882.
Timmusk, S., Grantcharova, N., & Wagner, E. G. H. (2005). Paenibacilluspolymyxa invades plant roots and forms biofilms. Applied and Environmental Microbiology, 71, 7292–7300.
Umesh, P. M., & Sanjay, R. M. (2012). Efficacy of bioagents and fungicides on seed mycoflora, germination and vigour index of cowpea. Science Research Reporter., 2(3), 321–326.
Umit, A. (2015). Evaluation of antifungal activity of mono and dipotassium phosphates against phytopathogenic fungi. Fresenius Environmental Bulletin., 24(3), 810–816.
Urrea, R., Cabezas, L., Sierra, R., Cardenas, M., Restrepo, S., & Jimenez, P. (2011). Selection of antagonistic bacteria isolated from the Physalisperuvianarhizosphere against Fusariumoxysporum. Journal of Applied Microbiology., 111, 707–716.
Wang, W., Fang, Y., Imran, M., Hu, Z., Zhang, S., Huang, Z., & Liu, X. (2021). Characterization of the Field Fludioxonil Resistance and Its Molecular Basis in Botrytis cinerea from Shanghai Province in China. Microorganisms., 9, 266.
Weber, B., & Halterman, D. A. (2012). Analysis of genetic and pathogenic variation of Alternariasolani from a potato production region. European Journal of Plant Pathology., 134, 847–858.
Weitang, S., Ligang, Z., Chengzong, Y., Xiaodong, C., Liqun, Z., & Xili, L. (2004). Tomato Fusarium wilt and its chemical control strategies in a hydroponic system. Crop Protection, 23(3), 120–123.
Xinyi, C., Yuanyuan, Z., Xuechi, F., Yan, L., & Qi, W. (2016). Isolation and characterization of Bacillus amyloliquefaciens PG12 for the biological control of apple ring rot. Postharvest Biology and Technology., 115, 113–121.
Yang, W., Xu, Q., Liu, H. X., Wang, Y. P., Wang, Y. M., Yang, H. T., & Guo, J. H. (2012). Evaluation of biological control agents against Ralstonia wilt on ginger. Biological Control., 62, 144–151.
Yazici, S., Yanar, Y., & Karaman, I. (2011). Evaluation of bacteria for biological control of early blight disease of tomato. African Journal of Biotechnology., 10(9), 1573–1577.
Yi-Chun, C., & Cheng-Hua, H. (2020). Biocontrol of bacterial spot on tomato by foliar spray and growth medium application of Bacillus amyloliquefaciens and Trichodermaasperellum. European Journal of Plant Pathology., 156, 995–1003.
Yurina, T. P., Karavaev, V. A., & Solntsev, M. K. (1993). Characteristics of metabolism in two cucumber cultivars with different resistance to powdery mildew. Russiun Plant Physiology, 40, 197–202.
Zhang, C., Imran, M., Liu, M., Li, Z., Gao, H., Duan, H., Zhou, S., & Liu, X. (2020a). Two Point Mutations on CYP51 Combined With Induced Expression of the Target Gene Appeared to Mediate Pyrisoxazole Resistance in Botrytis cinerea. Frontiers in Microbiology., 11, 1396.
Zhang, D., Yu, S., Yang, Y., Zhang, J., Zhao, D., Pan, Y., Fan, S., Yang, Z., & Zhu, J. (2020b). Antifungal Effects of Volatiles Produced by Bacillus subtilis Against Alternariasolani in Potato. Frontiers in Microbiology., 11, 1196.
Zhao, Y., Zhang, W., Xu, W., Mai, K., Zhang, Y., & Liufu, Z. (2012). Effects of potential probiotic Bacillus subtilis T13 on growth, immunity and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus. Fish & Shellfish Immunology, 32, 750–755.
Zhou, T. T., Chen, D., Li, C. Y., Sun, Q., Li, L. Z., Liu, F., Shen, Q., & Shen, B. (2012). Isolation and characterization of Pseudomonas brassicacearum J12 as an antagonist against Ralstoniasolanacearum and identification of its antimicrobial components. Microbiological Research., 167, 388–394.
Zhu, S. Y., & Hong, D. L. (2008). Comparison between two hybrid cultivars of indica rice (Oryza sativa L.) in seed vigor and biochemical traits after aging. Chinese Journal of Eco Agriculture., 16(2), 396–400.
Acknowledgements
The authors would like to thank Department of Arid Land Agriculture, Faculty of Meteorology,
Environment and Arid Land Agriculture for providing the lab facilities. We would like to express gratitude to the ministry of Education for providing scholarship for accomplishing the research work efficiently.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial,or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
KA-E, MI, MA and MMS the idea of the work and contributed to data curation and their validation as well as writing original draft. MI contributed to the formal analysis of the data all authors contributed to the reviewing and editing the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have anyactual or potential conflict of interest.
Ethical approval
Our manuscript is original research and itis not submitted to full or in parts to other journal for publication.
Research involved in human and animal rights
The research did not involve any studies with humanparticipants or animal as experimental model.
Informed consent
All authors havereviewed the manuscript and approved the final version of manuscriptbefore submission.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Imran, M., Abo-Elyousr, K.A.M., Mousa, M.A.A. et al. A study on the synergetic effect of Bacillus amyloliquefaciens and dipotassium phosphate on Alternaria solani causing early blight disease of tomato. Eur J Plant Pathol 162, 63–77 (2022). https://doi.org/10.1007/s10658-021-02384-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-021-02384-8