Abstract
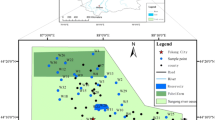
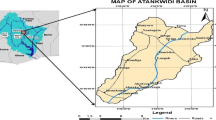
Hydrogeochemical studies were carried out in the Penna–Chitravathi river basins to identify and delineate the important geochemical processes which were responsible for the evolution of chemical composition of groundwater. The area is underlain by peninsular gneissic complex of Archaean age, Proterozoic meta-sediments, and strip of river alluvium. Groundwater samples were collected covering all the major hydrogeological environs in pre- and post-monsoon seasons. The samples were analyzed for major constituents such as Ca2 + , Mg2 + , Na + , K + , CO3 − , HCO3 − , Cl − , SO2 − 4, NO3 − , and F − . The groundwater in general is of Na + –Cl − , Na + –HCO3 − , Ca2 + –Mg2 + –HCO3 − , and Ca2 + –Mg2 + –Cl − types. Na + among cations and Cl − and/or HCO3 − among anions dominate the water; Na + and Ca2 + are in the transitional state with Na + replacing Ca2 + and HCO3 − Cl − due to physiochemical changes in the aquifer and water–rock interactions. The Ca2 + –Mg2 + –Cl − HCO3 − type water in one third samples suggest that ion exchange and dissolution processes are responsible for its origin. Change in storage of aquifer in a season does not influence the major geochemical makeup of groundwater. Gibbs plots indicate that the evolution of water chemistry is influenced by water–rock interaction followed by evapotranspiration process. The aquifer material mineralogy together with semiarid climate, poor drainage system, and low precipitation factors played major role in controlling groundwater quality of the area.
Similar content being viewed by others
References
Apadaca, L. E., Jeffrey, B. B., & Michelle, C. S. (2007). Water quality in shallow alluvium aquifers, Upper Calordo river basin, Calorado. Journal of the American Water Resources Association, 38(1), 133–148.
APHA (1995). Standard methods for the examination of water and wastewater (17th ed.). Washington, D.C.: American Public Health Association.
Beck, B. F., Asmussen, L., & Leonard, R. S. (1985). Relationship of geology, physiography, agricultural land use and groundwater quality in southwest Georgia. Groundwater GRWAAP, 23(5), 627–634.
Brijraj, K., & Kaur, P. (2007). Geochemistry of surface and sub-surface waters of Rawalyar lakes, Mandi district, Himachal Pradesh: Constraints on weathering and erosion. Journal of the Geological Society of India, 69, 1020–1030.
Cerling, T. E., Pederson, B. L., & Damn, K. L. V. (1989). Sodium-calcium ion exchange in the weathering of shales implication for global weathering budgets. Gelogy, 17, 552–554.
Chadha, D. K. (1999). A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeology Journal, 7, 431–439.
Datta, P. S., & Tyagi, S. K. (1996). Major ion chemistry of groundwater in Delhi area: Chemical weathering processes and groundwater flow regime. Journal of the Geological Society of India, 47, 179–188.
Doe, N.A., & Windecker, N. (2005). Groundwater notes. SHALE, 11, 37–44.
Domenico, P. A. (1972). Concepts and models in groundwater hydrology. New York: Mc Graw Hill.
Elango, L., & Kannan, R. (2007). Rock–water interaction and its control on chemical composition of groundwater. Chap. 11. Developments in Environmental Science, 5, 229–243, Publ. Elsevier.
Elango, L., Kannan, R., & Senthil Kumar, M. (2003). Major ion chemistry and identification of hydrogeochemical processes of groundwater in a part of Kancheepuram district, Tamil Nadu. Environmental Geosciences, 1(4), 157–166.
Fisher, R. S., & Mulican, III, W. F. (1997). Hydrogeochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the Northern Cnihvahvan desert. Trans-Pecos, Texas USA. Hydrogeology Journal, 10(4), 455–474.
Gibbs, R. J. (1970). Mechanisms controlling World’s water chemistry. Science, 170, 1088–1090.
Gopinath, G., & Seralathan, P. (2006). Chemistry of groundwater in the laterite formation of Muvatterpuzha river basin, Kerala. Journal of the Geological Society India, 68, 705–714.
Gowd, S. S. (2005). Assessment of groundwater quality for drinking and irrigation purposes: A case study of Peddavanka watershed, Anantapur District, Andhra Pradesh, India. Environmental Geology, 48, 702–712.
Gosselin, C. D., Edwin, H. F., & Flowerday, C. (2003). The complex Dakota aquifer: Managing groundwater in Nebraska. Geotimes April 2003.: http://www.copyright.com/ccc/do/showConfigurator?WT.mc_id=PubLink.
Govt. of Andhra Pradesh (2004). Handbook of statistics: Anantapur District. Andhra Pradesh: Govt. of Andhra Pradesh.
Hem, J. D. (1991). Study and interpretation of the chemical characteristics of natural water. US Geol Surv Water Supply Paper (3rd ed.). Jodhpur: Scientific.
Huh, Y., Tsoi, M. Y., Zaitiser, A., & Edward, J. N. (1998). The fluvial geochemistry of the river of Eastern Siberia, 1. Tributaries of Lena river drainage the sedimentation platform of the Siberia Craton. Geochimica et Cosmochimicha Acta, 62, 1657–1676.
Jankowski, J., & Acworth, R. I. (1997). Impact of depris-flow deposit on hydrogeochemical processes and the development of dry land salinity in the Yass River catchment, New South Wales, Australia. Hydrogeology Journal, 5(44), 71–88.
Johnson, C. C. (1979). Land application of water-an accident waiting to happen. Groundwater, 17(1), 69–72.
King, L. J., & Olsen, H. W. (1999). Hydraulic conductivity reductions resulting from clay dispersion within alluvial sediments impacted by sodium-rich water. US Geological Survey Toxic Substances Hydrology Program–Proceedings of the Technical Meeting Charleston South Carolina March 8–12, 1999–Volume 3 of 3–Subsurface Contamination From Point Sources, Water-Resources Investigations Report 99–4018C, http://toxics.usgs.gov/pubs/wri99–4018/Volume3/SectionE/3608_King/index.html.
Kumar, M., Ramanathan, A. L., Rao, M. S., & Kumar, B. (2006). Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi. Environmental Geology, 50, 1025–1039.
Lavitt, N., Acworth, R. I., & Jankowski, J. (1997). Vertical hydrogeochemical zonation in a coastal section of the Botany Sands aquifer, Sydney, Australia. Hyrogeology Journal, 5, 64–74.
Matthess, G. (1982). The properties of groundwater (p. 498). New York: Wiley.
May, A. L., & Loucks, M. D. (1995). Solute and isotope geochemistry and groundwater flow in the Central Wasatch Range, Utah. Journal of Hydrology, 170, 795–840.
McIntosh, J. C., & Walter, L. M. (2006). Paleowater in Silurian-Devonian carbonate aquifers: Geochemical evolution of groundwater in the Great Lakes region since Late Pleistocene. Geochimica Cosmochimica Acta, 70, 2454–2479.
Meybeck, M. (1987). Global chemical weathering of surficial rocks estimated from river dissolved leads. American Journal of Science, 287, 401–428.
Pandian, K., & Sankar, K. (2007). Hydro-geochemistry and groundwater quality in the Vaippar River Basin, Tamil Nadu. Journal of the Geological Society of India, 69, 970–982.
Pophare, M. A., & Dewalkar, S. M. (2007). Groundwater quality in eastern and south eastern parts of Rajura Tehsil, Chendrapur district, Maharashtra. Gondwana Geol Mazn Sp, 11, 119–129.
Raghunath, H. M. (1982). Groundwater (p. 456). New Delhi: Wiley.
Raju, J. (2007). Hydrogeochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah District, Andhra Pradesh, South India. Environmental Geology, 52, 1067–1074.
Raju, K. C. C., Kareemuddin, M. D., & Prabhakara, R. P. (1979). Operation Anantapur. Miscellaneous publication 47: Geological Survey of India.
Randall, M. G., Trivedi, D. P., Graham, J., Small, J. S., & Hughes, C. (1996). Mineralogical characterization of sediments at the Drigg low level radioactive waste disposal site and the influence on groundwater chemistry. © 1995–2009. Warrendale: Materials Research Society.
Rashid, U., & Izrar, A. (2007). Hydrchemical characteristics of groundwater in parts of Kushva-Yamuna basin, Muzaffarnagar district, UP. Journal of the Geological Society of India, 69, 970–982.
Reddy, A. G. S., Niranjan, K. K., & Venkat, R. D. (2008). Seasonal and temporal variations in groundwater quality of north eastern parts of aantapur district, AP. Journal of Applied Geochemistry, 10(1), 103–112.
Reddy, A. G. S., Niranjan, K. K., Subba, R. D., & Sambashiva, R. S. (2009). Assessment of nitrate contamination due to groundwater pollution in north eastern part of Anantapur District, AP India. Environmental Monitoring and Assessment, 148, 463–476. doi:10.1007/s10661-008-0176-y.
Reddy, N. B. Y., & Prasad, K. S. S. (2005). Hydro-geochemistry of groundwater in and around Tadpatri area, Anantapuir district, AP. Journal of Indian Association of Environmental Management, 32, 66–73.
Sami, K. (1992). Recharge mechanism and geochemical processes in a semi arid sedimentary basin, Eastern Cape, South Africa. Journal of Hydrology, 139, 27–48.
Sarin, M. M., Krishnaswmay, S., Dilli, K., Somayajulu, B. L. K., & Moore, W. S. (1989). Major ion chemistry of the Ganga-Brahmaputra river system: Weathering processes and fluxes to the bay of Bengal. Geochimica et Cosmochimica Acta, 53, 997–1009.
Sastry, J. C. V. (1994). Groundwater chemical quality in river basins, hydrogeochemical facies and hydrogeochemical modeling. In Lecture notes-refresher course conducted by School of Earth Sciences. Tamil Nadu, India: Bharathidasan University, Thiruchirapalli.
Schoeller, H. (1965). Hydrodynamique lans lekarst (ecoulemented emmagusinement). Actes Colloques Doubronik, I: AIHS et UNESCO, 3–20.
Schoeller, H. (1967). Qualitative evaluation of groundwater resources. In Methods and techniques of groundwater investigation and development. Water Research, Series-33: UNESCO (pp. 44–52).
Singh, P. K., Amrita, M., Dinesh, M., Vinod, K. S., & Singh, S. (2006). Evaluation of groundwater quality in northern Indo-Gangetic alluvium region. Environmental Monitoring and Assessment, 112, 211–230. doi:10.1007/s10661-006-0357-5.
Soltan, M. E. (1998). Characterization, classification and evaluation of some groundwater samples in Upper Epygt. Chemos, 37, 735–747.
Soltan, M. E. (1999). Evaluation of groundwater quality in Dakhla Oasis (Egyptian Western Desert). Evironmental Monitoring and Assessment, 57, 157–168.
SoCo (2009). Sustainable agriculture and soil conservation, soil degradation processes. Fact sheet no. 4, http://eusoils.jrc.ec.europa.eu/projects/soil_atlas/.
Stallard, R. F., & Edmond, J. M. (1983). Geochemistry of the Amazon, the influence of geology and weathering environment on the dissolved load. Journal of Geophysical Research, 88, 9671–9688.
Stocking, M. A. (2006). Catena of sodium-rich soil in Rhodesia. European Journal of Soil Science, 30(1), 139–146. Published Online: 28 Jul 2006.
Subbarao, N. (2006). Seasonal variation of ground\(\backslash \) water quality in a part of Guntur district, AP, India. Environmental Geology, 49, 413–429.
Wallick, E. I., & Toth, J. (1976). Methods of regional groundwater flow analysis with suggestions for the use of environmental isotope and hydrochemical data in groundwater hydrology (pp. 37–64). Vienna: IAEA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddy, A.G.S., Kumar, K.N. Identification of the hydrogeochemical processes in groundwater using major ion chemistry: a case study of Penna–Chitravathi river basins in Southern India. Environ Monit Assess 170, 365–382 (2010). https://doi.org/10.1007/s10661-009-1239-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-009-1239-4