Abstract
Coastal lagoons are important but sensitive environments, being transitional zones between land and sea. The Khnifiss lagoon is the most important desert wetland in Morocco, but little data have been produced concerning heavy metal geochemistry and enrichments in the sediments. Therefore, 26 surface sediments (15 intertidal and 11 subtidal) and 2 sediment cores were collected in 2016 and analyzed for a selection of heavy metals. The data were processed to assess the degree of contamination and the corresponding potential ecological risk, using several accumulation/enrichment indices, and the singular and multi-metal risk indices. Mean concentrations in the bottom layers of the two cores, dating from a pre-industrial age according to geochronological analysis, were used as the local geochemical background. The resulting values were on the whole lower than those reported for other areas of the northeastern coast of Morocco. Multivariate statistics were also applied to better understand relationships among variables (metals and other geochemical parameters) and to reveal similarities among sample groups. The results showed that, although the lagoon is not yet affected by significant anthropogenic influences, small enrichments can be recognized, especially for Ni and Cd. The cause may be related to the proximity to the main national highway, the vehicles and machinery used in the saltworks located in the area, and the small harbors used principally for fishing. In addition, industrial emissions from the Atlantic coast of Morocco and adjacent countries can be reasonably attributed as additional contributors to the enrichments. In terms of potential ecological risk, Cd shows the greatest impact compared to the other metals investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental studies on coastal lagoons and marine estuaries are of considerable importance because these environments are characterized by complex biogeochemical processes. In particular, sediments can become sinks for pollutants (including heavy metals and pesticides), and play a key role in contaminant remobilization in aquatic systems under favorable conditions (Navarrete-Rodríguez et al., 2020).
Trace elements, such as heavy metals, are naturally present in the environment since their origin is closely related to the local geological features. However, in recent decades, many types of anthropogenic activities, such as the use of gasoline that caused the release of high amounts of lead (Pb) in the second half of the last century, have become a significant source of heavy metal emissions. Even remote or protected areas, although not directly affected by anthropogenic emissions (no or little vehicular traffic, industrial activity, tourism, etc.) can be subject to enrichment, since heavy metals are also involved in long-range transport through particulate matter (PM10 and PM2.5) or aerosols (Popoola et al., 2018). Moreover, heavy metal contamination of coastal environments can affect the health of both aquatic organisms and humans (Mountouris et al., 2002), so monitoring activities should be conducted to preserve the original environmental status as much as possible (Mountouris et al., 2002).
Given that element content in sediments is variable depending on the parent material and land use, and not only as a consequence of anthropogenic inputs, the definition of site-specific background levels of relevant elements is essential to assess sediment contamination (Armiento et al., 2011).
Metal content from dated sediments reveal pre-anthropogenic contents and can be effectively used as site-specific reference background values. For aquatic sediments, the use of 210Pb originating from the decay of atmospheric 222Rn is a well-established methodology to estimate sediment ages and sedimentation rates (Kirchner, 2011).
In this paper, a geochemical investigation of sediments from Khnifiss Lagoon National Park was performed to evaluate the distribution of heavy metal content and anthropogenic contributions, and to estimate the potential ecological risk assessment. Since studies concerning heavy metal contamination in the region are still limited (Idardare et al., 2013; Tnoumi et al., 2021), this paper aims to contribute to improving the geochemical knowledge of the area.
Geoenvironmental setting
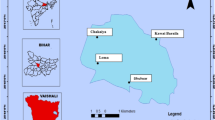
The Khnifiss lagoon (65 km2) is the most important desert wetland in Morocco and North Africa, located on the Atlantic Saharan coast (Fig. 1), in the SW of the country, approximately 120 km from the city of Tan-Tan and 70 km from Cape Juby (province of Tarfaya, region of Laayoune-Sakia El Hamra), within the Khnifiss National Park (a protected site and tourist destination of about 1850 km2). The area has been designated as a Ramsar site (area of relevance under the “Ramsar Convention on Wetlands of International Importance”) since 1980 and is part of the national network of protected areas as a SIBE site, Site d’Intérêts Biologique et Écologique (Benabid, 2000). To the south of Tan-Tan, an almost flat tabular surface develops, with a general slope on the order of 1% that gradually increases from west to east (Amimi et al., 2017). This extensive plateau, which expands over about 57% of the entire area, consists of a hard calcarenitic plate belonging to the Moghrébien (Upper Pliocene–Lower Pleistocene), which protects underlying marly-calcareous formations of Cretaceous age (Choubert et al., 1966). The area is also particularly relevant from an ecological point of view as it is a wintering site for birds during their migration.
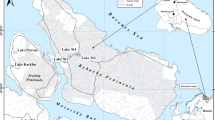
The lagoon is characterized by an average tidal range of about 2 m and receives freshwater from occasional rainfall and temporary underground and surface flows from the nearby Oued Aouedri river. The lagoon area consists of a large depression that extends for about 20 km within the coastline and is connected to the Atlantic Ocean by a large lagoon mouth named Foum Agoutir (Fig. 2). It is made of carbonate-based sediments deposited during the Upper Cretaceous (through marine transgressions) and Quaternary deposits of the coastal shelf. These deposits are separated from the Hamada Plateau (on the eastern and southern sides of the lagoon) by a rocky ridge, with an average elevation of 25 m, consisting of sandstones and conglomerates; in the western part and at the outlet, the lagoon is characterized by an impressive system of dunes (Dakki & Parker, 1988; El Agbani et al., 1988; Lakhdar Idrissi et al., 2004; Parker, 1988).
Materials and methods
Sampling and analysis
A total of 26 surface sediments were collected, as individual samples, during spring of 2016 (Fig. 2): 15 samples (from INT1 to INT15) were intertidal sediments, collected from the area that is submerged during high-tide periods; 11 samples (from SUB1 to SUB11) were subtidal sediments, sampled from the area that is always underwater. Samples were collected from a 0- to 5-cm depth using a stainless steel Van Veen grab sampler. Each sample was carefully taken from the central portion of the collected sediment with a plastic spatula previously washed with lagoon water, and then placed in pre-cleaned polyethylene bags. Two cores (KC1, from the downstream, and KC2, from the midstream of the lagoon) were also sampled to define the local background, by introducing a 50-mm-diameter PVC core sampler down to a depth of approximately 100 cm and 300 cm, respectively. The sampling sites were selected to avoid dredged areas and navigating channels, to minimize the risks of collecting disturbed sediments. At the laboratory, the cores were then subdivided into 2-cm subsamples from top to bottom. All samples were refrigerated at 4 °C until transferred to the analytical laboratory.
From each surface sediment sample, two aliquots were separated: one for geochemical analyses (major/trace element quantifications and particle size) and one for organic matter analysis. Aliquots for geochemical analyses were oven-dried at 40 °C, while those for organic matter determination were freeze-dried.
The oven-dried aliquots were sieved using a mechanical sieving device to obtain the fraction below 2 mm, which was used for geochemical analyses. Particle size analysis was performed using both dry and wet sieving techniques: samples were wet-separated into coarse and fine fractions using a 63-μm mesh sieve. The coarse fraction was dried again and then separated using ASTM series sieves. The wet fine fraction was analyzed using a SediGraph 5100 (Micromeritics) after being dispersed in a 0.5% sodium hexametaphosphate solution. Geochemical characterization was performed using a microwave-assisted acid digestion procedure according to EPA Method 3052, followed by ICP-MS analysis (PerkinElmer ELAN 6100), for trace elements (As, Cd, Co, Cr, Cu, Ni, Pb, V, Zn), and ICP-OES (PerkinElmer OPTIMA-2000 DV), for major elements (Al, Ca, Fe, K, Mg, Mn, Na, Ti). The quality of the analytical data was assessed using procedural blanks and by analyzing, with the same method adopted for the samples, two certified reference materials based on marine sediment (PACS-3 and MESS-4, National Research Council Canada, NRCC). Recoveries of Al, As, Cd, Co, Cr, Cu, Ni, Pb, V, and Zn ranged from 84 to 119% for MESS-4 and from 88 to 101% for PACS-3. Limits of quantitation (LOQs) were as follows: As, Co, Pb, and V = 0.05 mg/kg; Cd = 0.03 mg/kg; Cu and Ni = 0.3 mg/kg; Cr = 0.5 mg/kg; Mn and Zn = 1 mg/kg; Fe = 5 mg/kg; Ca, Mg, and Ti = 10 mg/kg; Na = 20 mg/kg; and Al and K = 50 mg/kg (Tnoumi et al., 2021). Organic matter (OM), which in lagoon sediments comes from both marine and terrestrial sources, was determined by the loss on ignition (LOI) procedure (Heiri et al., 2001).
Radiometric analyses were also performed by gamma spectrometry. Each sample was counted for 2 days on high-purity germanium detectors to determine 210Pb, 226Ra, and 137Cs activities. The excess 210Pb activity (210Pbex) was calculated as the difference between the total 210Pb and 226Ra. Details on calibrations, quality checks, and measurements have been presented elsewhere (Delbono et al., 2016). The 210Pb dating method has been widely used to establish geochronology in marine sediments on timescales of ~110 years (Sanchez-Cabeza & Ruiz-Fernández, 2012, and references therein). This method is based on the decay of the unsupported 210Pbex fraction present in the sediment and must always be verified using an independent temporal marker (Smith, 2001). For this purpose, 137Cs is frequently used; this artificial radionuclide was introduced into the environment primarily during atmospheric nuclear weapon testing (NWT) until 1963, when these tests were banned. To achieve dating, the constant rate of supply (CRS) model (Appleby & Oldfield, 1978; Robbins et al., 1977) was applied to the KC1 sediment core, assuming a constant flux of 210Pbex at the sediment–water interface and a variable mass accumulation rate (MAR, g/(cm2 year)). Due to irregularities in deposition, proper dating could not be established in core KC2.
Data elaboration
Several data elaboration techniques were used to estimate metal pollution in sediments, including general and multivariate statistics. In addition, a selection of indices was applied to assess the degree of contamination and the corresponding ecological risk.
Background matrix
To properly interpret geochemical data, selecting a background matrix is an essential step in highlighting enrichments/dissimilarities. Commonly used background matrices are the “average shale” or “average upper continental crust,” but the availability of a local background is undoubtedly recommended and should be employed. The selection of the most appropriate matrix is a critical issue since discordant results and assessments can be obtained utilizing different background data. In this work, the two abovementioned local sediment cores were used to define the background matrix; indeed, considering both the heavy metals’ depth-profile results and the chronological dating, some layers from the lower segments of the sediment cores (2 layers from KC1 and 3 layers from KC2), dating back to about 100 years and beyond, were evaluated as pristine and the element averages were used as the local geochemical background (LGB).
Data normalization
Before the data processing and interpretation using multivariate statistics, normalization was applied. This preliminary step is necessary because metals in sediments can derive from both natural sources (such as bedrock material) and anthropogenic sources. Therefore, to assess the degree of contamination, it is important to differentiate the actual contribution from these origins. Input from natural sources could differ by several orders of magnitude, depending on mineralogy and particle size distribution (Loring, 1991), and could lead to an overestimation of the anthropogenic contribution. Consequently, a normalization procedure should be used to compensate for particle size and mineralogical effects on metal content.
A typical geochemical approach is the normalization of metal data based on the content of a conservative element (Loring, 1990; Schropp & Windom, 1988). Among the parameters in our database, Al, Fe, and Ti showed very good correlations with the other elements (both major and trace) and could be used as normalizers. However, Al exhibited the best correlation (r = 0.87) with the fine fraction (silt + clay), and also high correlations with all heavy metals (r values ranging from 0.68 to 0.89). Therefore, it was judged to be the most appropriate for the Khnifiss lagoon sediments.
Assessment of sediment enrichment and potential ecological effects
The degree of heavy metal enrichment in the surface sediments, and the related potential ecological effects, were estimated by a selection of indices (Table 1) calculated using the abovementioned LGB as background values: the enrichment factor (EF) (Grant & Middleton, 1990; Salomons & Forstner, 1984; Woitke et al., 2003), the geoaccumulation index (Igeo) (Muller, 1969; Ntekim et al., 1993), the pollution load index (PLI) (Tomlinson et al., 1980), the modified degree of contamination (mCd) (Abrahim & Parker, 2008), and the risk index (RI) (Hakanson, 1980).
Results and discussion
Core geochronological dating
In the KC1 sediment core, the CRS age model allowed estimation of the age of the layers up to the equilibrium depth, where 210Pbex falls to zero (Fig. 3a). The vertical profile of 210Pbex (Fig. 3b) did not show regular exponential decay, thus confirming that constant sedimentation rate could not be assumed. The deep maximum of 137Cs was observed in the 46–48-cm layer, in agreement with the calculated age (1968 ± 2). Considering that not all layers were analyzed, this can be considered the required confirmation of 210Pb dating. It should be noted, however, that 137Cs did not disappear in the layers older than 1954, and this is a feature related to the fraction of 137Cs that is diffuse in interstitial water (Abril et al., 1991). The CRS model also allowed MAR to be estimated for each layer: in this core, MAR was higher in layers younger than 1968 (mean value = 1.2 ± 0.4 g/(cm2 year) compared with 0.5 ± 0.2 g/(cm2 year) in the older layers), comparable with values in other lagoons in Morocco (Benmhammed et al., 2021; Maanan et al., 2013, 2014).
In the KC2 sediment core, the 210Pbex and 137Cs profiles did not yield a confirmed dating. However, it was possible to conclude that the layers in which 210Pbex and 137Cs are negligible (deeper than 70 cm) had no relevant input from the surface during the last 110 years (Fig. 3c).
The use of sediment cores provides not only background levels, but also produces a record of contamination and when dated, as in this case, establishes the age of onset of pollution (Birch, 2017).
General statistics
The general statistics of the analysis results are summarized in Table 2 (for intertidal sediments) and Table 3 (for subtidal sediments). The average concentrations of major elements in surface sediments were lower than those reported for the upper continental crust (UCC; Rudnik & Gao, 2014), except for Ca, which showed quite higher amounts in both intertidal and subtidal sediments (Table 4) that may be related to eolian and biogenic inputs (Tnoumi et al., 2021). The distribution of OM was heterogeneous, showing significant variation between intertidal (higher values) and subtidal (lower values) samples. The OM content in sediments has many sources such as terrigenous deposits, marine deposits, and biota decomposition (Bentivegna et al., 2004). However, the state of the lagoon and the absence of terrestrial inputs suggest that marine productivity is the primary source of OM in this area (Tnoumi et al., 2021).
Heavy metal concentrations in surface sediments showed, on the whole, the following sequences: Mn > V > Cr > Zn > Ni > As > Pb > Cu > Co > Cd (for intertidal samples) and Mn > V > Ni > Cr > Zn > As > Cu > Pb > Co > Cd (for subtidal samples). In addition, for all metals investigated, trends showed decreasing concentration levels as sediment grain size increased. Our results are in excellent agreement with the data produced by Idardare et al. (2013), for intertidal sediment samples from the same area (Table 4).
The coefficient of variation for heavy metals ranged from 14.9 to 45.5% for the intertidal samples and from 12.2 to 62.2% for the subtidal samples. In particular, As, Cd, Cr, and Zn, for intertidal sediments, and all heavy metals except As, for subtidal sediments, were characterized by variation greater than 30%, showing considerable spatial variability. Such outcomes imply that heavy metal occurrences at each site are potentially affected by several factors including human activities.
The sediment cores exhibited decreasing trends from top to bottom in metal content. The bottom layers were datable before the onset of massive anthropogenic inputs (pre-industrial age). This confirmation allowed us to hypothesize that the average element concentrations of the bottom layers can be considered as the LGB of the area. The resulting sequence of heavy metal concentrations is Mn > V > Cr > Zn > Ni > As > Pb > Cu > Co > Cd, which is very similar to that obtained for the surface samples. This is a first hint suggesting that the lagoon sediments are not significantly affected by contamination. The LGB data for the Khnifiss lagoon are reported in Table 4, where local background values for other Moroccan lagoons acquired from various sources and the aforementioned UCC and average shale are also summarized.
Multivariate statistics analysis
To evaluate the data in their wholeness, a multivariate statistical approach was applied. Principal component analysis (PCA) and cluster analysis were performed on the complete surface sediment dataset. According to literature, multivariate statistics produces reliable results when executed on a sufficiently large dataset (e.g., Comrey and Lee (1992) suggested that a reasonable size is about 200 samples), or on a dataset with a certain minimum sample to variable ratio (Cattell (1978) recommended a ratio of 3:1). Therefore, being aware that due to the small size of the dataset proposed in this article, and the not optimal cases/variables ratio, multivariate statistics cannot be performed rigorously; the results proposed here must be intended as merely indicative.
Bartlett’s sphericity test and the Kaiser–Meyer–Olkin index (0.001 and 0.72 respectively, p < 0.01) indicated that PCA is suitable on this dataset as a reduction tool. The first two PCA components obtained explain about 80% of the entire dataset variance. The first factor (accounting for about 59% of the dataset total variance) is mainly driven by Al, Mg, Ti, Fe, Na, K, OM, fine fraction (silt + clay), and most of the heavy metals. Their significant positive correlations might represent common sources for these metals. The contribution of Al, K, and Fe, in particular, may imply the role of clay minerals and Al and Fe oxy-hydroxides in the adsorption of heavy metals. Moreover, the positive correlation between OM and the abovementioned parameters also suggests the OM contribution in the accumulation of heavy metals in the sediments. Factor 2 (accounting for about 22% of the total variance) is mainly characterized by Mn, As, Cu, Pb, V, Cr, Zn, and Co. These elements showed a moderate mutual correlation, assuming their sources from various local pollution such as (i) vehicular traffic along the nearby highway; (ii) saltworks activities and related heavy machines – the sebkha Tazera contributes with other sebkhas about 20,000 tons/year of salt production; and (iii) boats used for tourism and fishing activities. Pb is a marker of vehicular traffic emissions which contributes more than 50% in the metal’s total release (Pulles et al., 2012). Zn is reasonably associated with the combustion of lubricating oil, and the highest levels have been found near the wood docks and the salt pans, while Cr and Cu are the markers of antifouling paints (Pulles et al., 2012).
The resulting PCA scatter plot (Fig. 4) showed that the lagoon sediments can be gathered into two groups whose differentiation is mainly related to the amount of OM and fine fraction. Indeed, “group 1” includes the samples characterized by the lowest levels of trace metals, OM, and fine fraction (silt + clay) in the dataset; these parameters are closely related since OM contains functional groups that can form complexes with trace metals, and the fine fraction has a higher surface area and adsorption capacity (Fernandes et al., 2011; Sadeghi et al., 2012). In contrast, “group 2” includes samples with the highest values of trace metals, OM, and fine fraction. Furthermore, each group can be split into two further subcategories characterized by minor differences: the subcategory of group 1 that includes the INT5 and SUB8 samples is particularly interesting since the orientation of the PCA vectors and the substantial distance of the samples from the other subcategory are indications of relevant differentiation in terms of metal enrichment.
The PCA also highlighted the existing geochemical distinction between intertidal and subtidal sediments, as they proved to be well distinguishable in the two groups: indeed, except for samples INT1 and INT5, all intertidal samples were part of group 2, while all subtidal samples were included in group 1.
Cluster analysis confirmed the PCA results since the same groups and subgroups are clearly distinguished (Fig. 5).
Assessment of the enrichment degree
Although multivariate statistics highlighted some samples exhibiting signs of enrichment, and a group of samples with higher trace element concentration levels compared to the others, it is not sufficient to assess the state of the lagoon sediments. Indeed, it is necessary to compare the data with a reference matrix to evaluate whether the presumed metal enrichments are significant or negligible.
Enrichment factor (EF) results
The results of the EF calculations are arranged as electronic supplementary material in Table ESM1. According to the EF guideline interpretation proposed by many authors (e.g., Chen et al. (2007)), metal contamination can be classified into the following categories: EF ≤ 1, no enrichment; 1 < EF ≤ 3, minor enrichment; 3 < EF ≤ 5, moderate enrichment; 5 < EF ≤ 10, moderately severe enrichment; 10 < EF ≤ 25, severe enrichment; 25 < EF ≤ 50, very severe enrichment; and EF > 50, extremely severe enrichment. The results found on the Khnifiss surface sediments showed no enrichment or evidenced only minor enrichments. The highest values were found for Ni (3.0) in sample SUB3 and Cd (2.6) in sample INT12. The elements that showed the most occurrences of “minor enrichment” were Ni (20 samples), Cu (18 samples), and V (15 samples). Sediments INT5 and SUB8 are those that especially displayed an overall sign of “minor enrichment,” due to the higher incidence of elements having EF > 1, and also evidenced by the highest mean EF values (1.6 ± 0.5 and 1.6 ± 0.4, respectively); this finding confirms the results of the multivariate statistics since these two samples are the same belonging to the abovementioned subcategory of group 1 in the PCA.
For comparison purposes, we also calculated the EFs using the widely adopted “average shale composition” as background values (Turekian & Wedepohl, 1961), and then matched the results with those obtained from LGB. As reported in Table 5, the EFs determined using the shale data are sensibly higher for As, Cd, and Cr. In particular, for Cr, the values denote a condition of “moderately severe enrichment” for intertidal samples and “moderate enrichment” for subtidal samples (two categories and one category higher than the “minor enrichment” resulting from the EFs obtained using the local background, respectively). In contrast, EFs for Co, Cu, Mn, Ni, Pb, and Zn are higher when calculated using LGB. This comparison underlines the importance of a wise and proper selection of the reference matrix used in the EF calculation and the importance of the availability of local background values, to avoid overestimates/underestimates and thus inaccurate classifications that do not reflect the true environmental context.
Geoaccumulation index (Igeo) results
The Igeo results are collected as electronic information in Table ESM2 and are classified according to the following criteria (commonly acknowledged by many authors, such as Nilin et al. (2013)): Igeo ≤ 0, uncontaminated; 0 < Igeo ≤ 1, uncontaminated to moderately contaminated; 1 < Igeo ≤ 2, moderately contaminated; 2 < Igeo ≤ 3, moderately to heavily contaminated; 3 < Igeo ≤ 4, heavily contaminated; 4 < Igeo ≤ 5, heavily to extremely contaminated; and Igeo > 5, extremely contaminated. The Igeo scores showed a good agreement with the EF results in terms of the degree of enrichment: the values for the various elements, on the whole, fall within the first two lowest Igeo categories, depicting a condition of limited contamination. The highest score, corresponding to “uncontaminated to moderately contaminated” sediments, was concerning Cd (1.3) for sample INT12 (a sample that also draws our attention in the discussion of EFs).
However, it is important to note that the Igeo scores evidenced more uncontaminated samples than EF. Indeed, several sediments, especially among the subtidal ones, that were classified as having “minor enrichments” according to EF are instead rated as uncontaminated by Igeo. This discordance between Igeo and EF classification was predictable, as already reported by many authors (e.g., Haris and Aris (2013); Loska et al. (2003); Nowrouzi and Pourkhabbaz (2014); Shafie et al. (2013); Sukri et al. (2018)). In fact, it is due to the normalization step implied in the EF formula, and particularly related to the choice of the reference element and the background matrix. Therefore, even considering that the normalization step of the EF was based on the local geochemical background values, the Igeo evaluation appears to be less reliable in the studied area.
Multielement contamination assessment results
The PLI results are shown in Fig. 6. Conversely to the previously discussed single element–based indices, PLI is multielement-based, accounting for the contribution of several heavy metals. In this case, PLI was calculated considering the five potentially most hazardous heavy metals (As, Cd, Cu, Ni, Pb) for the Khnifiss lagoon. According to Tomlinson et al. (1980), PLI > 1 values indicate sample stations that can be considered polluted, whereas if PLI < 1, there is a low level of metal pollution. All intertidal samples except INT1 showed a PLI greater than 1. The highest value was calculated for INT4 (PLI = 2.16). Thus, notwithstanding that from the previous indices the lagoon sediments are only characterized by minor enrichments for some elements, PLI also showed that from a general point of view, the intertidal samples are affected by pollutant inputs. Considering subtidal sediments, only the SUB2 sample exhibited a PLI greater than 1. Moreover, the differences between intertidal and subtidal sediments suggested that tidal cycles play a relevant role in pollutant transport and accumulation.
Considering the surface sediment data, which represent the current heavy metal amount in the area, and LGB, indicating the natural geogenic occurrence of the elements, an attempt to display the overall spatial distribution of the heavy metal enrichments was made. A map based on mCd (Fig. 7) confirms again that only a very low or low heavy metal enrichment affects the lagoon. Furthermore, it also evidences that the contamination is distributed in the entire lagoon area but only in the intertidal sediments. Only two points showed moderate contamination, located in the lagoon’s mouth (sample INT4), where the tidal influence is stronger, and in the inner section (sample INT14), where the saltworks-related contribution is closer.
Potential ecological risk index (RI) results
According to the results of the contamination factor (µCF), which is the starting parameter for the calculation of ecological risk, the following sequence was highlighted (Table 6): Ni > Cu > V > As > Cr > Cd > Co = Pb > Zn > Mn. Intertidal samples were found to be more sensitive to contamination than subtidal samples (1 ≤ µCF < 3, corresponding to moderate contamination, and µCF < 1, corresponding to low contamination).
The potential ecological risk posed by the investigated elements to the lagoon ecosystem is negligible (RI always less than 150). The risk factor (Er) associated with each individual heavy metal was classified as “low” (Er < 40), with the exception of Cd in intertidal sediments, which was rated as “moderate.” Er values followed the progression Cd > As > Ni > Cu > Co > Pb > V > Cr > Zn > Mn.
Table 7 reports the results of the potential ecological risk posed by Cd, Cr, Cu, Mn, Ni, Pb, and Zn, estimated for the five lagoons located along the Atlantic coast of Morocco (Khnifiss and other four sites). In order to calculate the risk indices, average surface sediment metal concentrations taken from the literature (Boutahar et al. (2019) and Maanan et al. (2015)) and background concentrations relative to each lagoon previously reported in Table 4 were used. As and Co were not considered in this elaboration, due to the absence of data in some lagoons.
The results showed that Khnifiss and Merja Zerga lagoons can be associated with low risk (with slightly higher risk for Khnifiss), while Sidi Moussa, Nador, and Oualidia with moderate risk. In all four lagoons, the main contribution to ecological risk can be ascribed mainly to Cd. Only in the case of Merja Zerga did the factors show that Ni has greater relevance than Cd on the risk affecting the ecosystem.
Conclusions
Surface sediments from the Khnifiss lagoon were analyzed to assess enrichment in heavy metals. The geochronological investigation of two sediment cores from the same area, dated by 210Pb and 137Cs, allowed to identify pre-industrial layers and ultimately to calculate site-specific background concentrations. The obtained data were used in this study as reference values in the calculation of an assortment of contamination indices, but also represent an important resource for future investigations focused on the time trend of the anthropogenic heavy metal enrichment in areas with similar geomorphological and geochemical characteristics.
The results showed that the area is affected by scarce anthropogenic impact, and only minor/moderated enrichments were detected. The sources of metals may be related to the various human activities in the proximity of the lagoon, in particular vehicular traffic associated with the adjacent National Route 1 (N1) highway, which runs alongside the Khnifiss National Park; motor fishing boats in the small harbors also located in the lagoon area; tourist vehicles circulating in the lagoon region; and machinery and trucks used in the extensive saltworks operating in the southern portion of the lagoon. Transport of contaminants related to nearby Tarfaya (the closest town to Khnifiss National Park) and the Canary Islands, and industrial emissions from the Atlantic coast of Morocco and western Algeria are also plausible contributors to enrichment. Intertidal sediments appeared to be more exposed to the heavy metal inputs, indicating a relevant role of tidal cycles in contaminants’ transport and deposition. According to the comparison with the other four Moroccan lagoons, Khnifiss showed a lower ecological risk.
In order to ensure proper environmental management of the Khnifiss lagoon, we suggest to periodically repeat the sampling campaign and to extend the investigation by including samples from areas not yet investigated.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
23 July 2022
Missing Open Access funding information has been added in the Funding Note.
References
Abrahim, G. M. S., & Parker, R. J. (2008). Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environmental Monitoring and Assessment, 136(1), 227–238.
Abril, J., García-León, M., García-Tenorio, R., Sánchez, C., & El-Daoushy, F. (1991). Dating of marine sediments by an incomplete mixing model. Journal of Environmental Radioactivity, 15(2), 135–151.
Amimi, T., Ouhnine, R., Chao, J., Elbelrhiti, H., & Koffi, A. S. (2017). Potentialités de l’écotourisme et géotourisme aux provinces de Tantan, Tarfaya et Layoune (Sahara Atlantique marocain). European Scientific Journal, 13(15), 133–147.
Appleby, P. G., & Oldfield, F. (1978). The calculation of lead-210 dates assuming a constant rate of supply of unsupported 210Pb to the sediment. CATENA, 5(1), 1–8.
Armiento, G., Cremisini, C., Nardi, E., & Pacifico, R. (2011). High geochemical background of potentially harmful elements in soils and sediments: Implications for the remediation of contaminated sites. Chemistry and Ecology, 27(1), 131–141.
Benabid, A. (2000). Flore et écosystèmes du Maroc. Ibis press, Paris.
Benmhammed, A., Laissaoui, A., Mejjad, N., Ziad, N., Chakir, E., Benkdad, A., et al. (2021). Recent pollution records in Sidi Moussa coastal lagoon (western Morocco) inferred from sediment radiometric dating. Journal of Environmental Radioactivity, 227, 106464.
Bentivegna, C. S., Alfano, J.-E., Bugel, S. M., & Czechowicz, K. (2004). Influence of sediment characteristics on heavy metal toxicity in a urban mash. Urban Habitats, 2(1), 91–111.
Birch, G. F. (2017). Determination of sediment metal background concentrations and enrichment in marine environments - A critical review. Science of the Total Environment, 580, 813–831.
Boutahar, L., Maanan, M., Bououarour, O., Richir, J., Pouzet, P., Gobert, S., Maanan, M., Zourarah, B., Benhoussa, A., & Bazairi, H. (2019). Biomonitoring environmental status in semi-enclosed coastal ecosystems using Zostera noltei meadows. Ecological Indicators, 104, 776–793.
Cattell, R. B. (1978). The scientific use of factor analysis in behavioral and life sciences. Plenum.
Chen, C. W., Kao, C. M., Chen, C. F., & Di Dong, C. (2007). Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbour, Taiwan. Chemosphere, 66, 1431–1440.
Choubert, G., Faure-Muret, A., & Hottinger, L. (1966). Aperçu géologique du bassin côtier de Tarfaya. Notes Et Mémoires Du Service Géologique, 175(1), 12–33.
Comrey, A. L., & Lee, H. B. (1992). A first course in factor analysis (2nd ed.). Lawrence Erlbaum Associates.
Dakki, M., & Parker, D. M. (1988). The Khnifiss Lagoon and adjacent desert areas: Geographical description. In M. Dakki & W. De Ligny (Eds.), The Khnifiss Lagoon and its surrounding environment (Province of La’yyoune, Morocco) (pp. 165–172). Rabat: Travaux de l’Institut Scientifique mem hors serie.
Delbono, I., Barsanti, M., Schirone, A., Conte, F., & Delfanti, R. (2016). 210Pb mass accumulation rates in the depositional area of Magra River (Mediterranean Sea, Italy). Continental Shelf Research, 124(1), 35–48.
El Agbani, M. A., Ferkhaoui, M., Bayed, A., & Schouten, J. R. (1988). The Khnifiss Lagoon and adjacent waters: Hydrology and hydrodynamics. In M. Dakki & W. De Ligny (Eds.), The Khnifiss Lagoon and its surrounding environment (Province of La’yyoune, Morocco) (pp. 18–27). Rabat: Travaux de l’Institut Scientifique mem hors serie.
Fernandes, L., Nayak, G. N., Ilangovan, D., & Borole, D. V. (2011). Accumulation of sediment, organic matter and trace metals with space and time, in a creek along Mumbai coast, India. Estuarine Coastal and Shelf Science, 91, 388–399.
Grant, A., & Middleton, R. (1990). An assessment of metal contamination of sediments in the Humber estuary, U.K. Estuarine Coastal and Shelf Science, 31, 71–85.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A Sedimentological Approach. Water Research, 14(8), 975–1001.
Haris, H., & Aris, A. Z. (2013). The geoaccumulation index and enrichment factor of mercury in mangrove sediment of Port Klang, Selangor, Malaysia. Arabian Journal of Geosciences, 6(11), 4119–4128.
Heiri, O., Lotter, A. F., & Lemcke, G. (2001). Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. Journal of Paleolimnology, 25, 101–110.
Idardare, Z., Moukrim, A., Chiffoleau, J.-F., Ait Alla, A., Auger, D., & Rozuel, E. (2013). Evaluation de la contamination métallique dans deux lagunes marocaines: Khnifiss et Oualidia. Revue Marocaine Des Sciences Agronomiques Et Vétérinaires, 2, 58–67.
Kirchner, G. (2011). 210Pb as a tool for establishing sediment chronologies: Examples of potentials and limitations of conventional dating models. Journal of Environmental Radioactivity, 102(5), 490–494.
Lakhdar Idrissi, J., Orbi, A., Zidane, F., Hilmi, K., Sarf, F., Massik, Z., & Makaoui, A. (2004). Organization and functioning of a Moroccan ecosystem: Khnifiss lagoon. Revue Des Sciences De L’eau, 17(4), 447–462.
Loring, D. H. (1990). Lithium — A new approach for the granulometric normalization of trace metal data. Marine Chemistry, 29, 155–168.
Loring, D. H. (1991). Normalization of heavy-metal data from estuarine and coastal sediments. ICES Journal of Marine Science, 48, 101–115.
Loska, K., Wiechuła, D., Barska, B., Cebula, E., & Chojnecka, A. (2003). Assessment of arsenic enrichment of cultivated soils in Southern Poland. Poland Journal of Environmental Studies, 12(2), 187–192.
Maanan, M., Landesman, C., Maanan, M., Zourarah, B., Fattal, P., & Sahabi, M. (2013). Evaluation of the anthropogenic influx of metal and metalloid contaminants into the Moulay Bousselham lagoon, Morocco, using chemometric methods coupled to geographical information systems. Environmental Science and Pollution Research, 20, 4729–4741.
Maanan, M., Ruiz-Fernandez, A. C., Maanan, M., Fattal, P., Zourarah, B., & Sahabi, M. (2014). A long-term record of land use change impacts on sediments in Oualidia lagoon, Morocco. International Journal of Sediment Research, 29(1), 1–10.
Maanan, M., Saddik, M., Maanan, M., Chaibi, M., Assobhei, O., & Zourarah, B. (2015). Environmental and ecological risk assessment of heavy metals in sediments of Nador lagoon, Morocco. Ecological Indicators, 48, 616–626.
Mejjad, N., Laissaoui, A., El-Hammoumi, O., Fekri, A., Amsil, H., El-Yahyaoui, A., & Benkdad, A. (2018). Geochemical, radiometric, and environmental approaches for the assessment of the intensity and chronology of metal contamination in the sediment cores from Oualidia lagoon (Morocco). Environmental Science and Pollution Research, 25(23), 22872–22888.
Mountouris, A., Voutsas, E., & Tassios, D. (2002). Bioconcentration of heavy metals in aquatic environments: The importance of bioavailability. Marine Pollution Bulletin, 44(10), 1136–1141.
Muller, G. (1969). Index of geoaccumulation in sediments of the Rhine river. Journal of Geology, 2, 108–118.
Navarrete-Rodríguez, G., Castañeda-Chávez, M. R., & Lango-Reynoso, F. (2020). Geoacumulation of heavy metals in sediment of the fluvial–lagoon–deltaic system of the Palizada River, Campeche, Mexico. International Journal of Environmental Research and Public Health, 17(3), 969.
Nilin, J., Moreira, L. B., Aguiar, J. E., Marins, R., de Souza, M., Abessa, D., da Cruz, M., Lotufo, T., & Costa-Lotufo, L. V. (2013). Sediment quality assessment in a tropical estuary: The case of Ceará River, Northeastern Brazil. Marine Environmental Research, 91, 89–96.
Nowrouzi, M., & Pourkhabbaz, A. (2014). Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Hara Biosphere Reserve. Chemical Speciation and Bioavailability, 26(2), 99–105.
Ntekim, E. E. U., Ekwere, S. J., & Ukpong, E. E. (1993). Heavy metal distribution in sediments from Calabar River, southeastern Nigeria. Environmental Geology, 21, 237–241.
Parker, D. M. (1988). Soils of the coastal platform between the Khnifiss and adjacent waters. In M. Dakki & W. De Ligny (Eds.), The Khnifiss Lagoon and its surrounding environment (Province of La’yyoune, Morocco) (pp. 8–17). Rabat: Travaux de l’Institut Scientifique mem hors serie.
Popoola, L. T., Adebanjo, S. A., & Adeoye, B. K. (2018). Assessment of atmospheric particulate matter and heavy metals: A critical review. International Journal of Environmental Science and Technology, 15, 935–948.
Pulles, T., Denier van der Gon, H., Appelman, W., & Verheul, M. (2012). Emission factors for heavy metals from diesel and petrol used in European vehicles. Atmospheric Environment, 61, 641–651. https://doi.org/10.1016/j.atmosenv.2012.07.022
Robbins, J. A., Krezoski, J. R., & Mozley, S. C. (1977). Radioactivity in sediments of the Great Lakes: Post-depositional redistribution by deposit-feeding organisms. Earth and Planetary Science Letters, 36(2), 325–333.
Rudnik, R. L., & Gao, S. (2014). Composition of the continental crust. In H. D. Holland & K. K. Turekian (Eds.), Treatise on geochemistry Chapter 1 (2nd ed., Vol. 4). Elsevier.
Sadeghi, S. H. R., Harchegani, M., & Younesi, H. A. (2012). Suspended sediment concentration and particle size distribution, and their relationship with heavy metal content. Journal of Earth System Science, 121, 63–71.
Sanchez-Cabeza, J. A., & Ruiz-Fernández, A. C. (2012). 210Pb sediment radiochronology: An integrated formulation and classification of dating models. Geochimica Et Cosmochimica Acta, 82, 183–200.
Salomons, W., & Forstner, U. (1984). Metals in the hydrocycle (p. 349). Springer-Verlag.
Schropp, S. J., & Windom, H. L. (1988). A guide to the interpretation of metal concentrations in estuarine sediments (p. 53). Georgia: Savannah.
Shafie, N. A., Aris, A. Z., Zakaria, M. P., Haris, H., Wan, Y. L., & Isa, N. M. (2013). Application of geoaccumulation index and enrichment factors on the assessment of heavy metal pollution in the sediments. Journal of Environmental Science and Health Part A – Toxic/Hazardous Substances & Environmental Engineering, 48(2), 182–190.
Smith, J. N. (2001). Why should we believe 210Pb sediment geochronologies? Journal of Environmental Radioactivity, 55(2), 121–123.
Sukri, N. S., Aspin, S. A., Kamarulzaman, N. L., Jaafar, N. F., Rozidaini, M. G., Shafiee, N. S., Yaacob, S. H., Kedri, F. K., & Zakaria, M. P. (2018). Assessment of metal pollution using enrichment factor (EF) and pollution load index (PLI) in sediments of selected Terengganu rivers, Malaysia. Malaysian Journal of Fundamental and Applied Sciences, 14(2), 235–240.
Tnoumi, A., Angelone, M., Armiento, G., Caprioli, R., Crovato, C., De Cassan, M., Montereali, M. R., Nardi, E., Parrella, L., Proposito, M., Spaziani, F., & Zourarah, B. (2021). Assessment of trace metals in sediments from Khnifiss Lagoon (Tarfaya, Morocco). Earth, 2, 16–31.
Tomlinson, D. L., Wilson, J. G., Harris, C. R., & Jeffrey, D. W. (1980). Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgolander Meeresuntersuchungen, 33, 566–575.
Turekian, K. K., & Wedepohl, K. H. (1961). Distribution of the elements in some major units of the Earth’s crust. Geological Society of American Bulletin, 72(2), 175–192.
Woitke, P., Wellmitz, J., Helm, D., Kube, P., Lepom, P., & Litheraty, P. (2003). Analysis and assessment of heavy metal pollution in suspended solids and sediments of the river Danube. Chemosphere, 51, 633–642.
Acknowledgements
The first author (A.T.) is deeply thankful to ENEA for providing all necessary research facilities, and greatly acknowledges the fellowship from the ICTP Programme for Training and Research in Italian Laboratories (Trieste, Italy). The authors also thank the “Haut Commissariat aux Eaux et Forêts et à la Lutte Contre la Désertification” for giving access during the sampling campaign. They are also delighted to express their gratitude and sincere thanks to the editor and the reviewers for their useful comments and suggestions to improve the quality of this paper.
Funding
Open access funding provided by Ente per le Nuove Tecnologie, l'Energia e l'Ambiente within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tnoumi, A., Angelone, M., Armiento, G. et al. Heavy metal content and potential ecological risk assessment of sediments from Khnifiss Lagoon National Park (Morocco). Environ Monit Assess 194, 356 (2022). https://doi.org/10.1007/s10661-022-10002-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10002-1