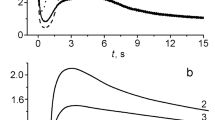
Abstract
The electrochemical ion-exchange properties of RuO2–TiO2 film electrodes with different composition have been studied in acidic and alkaline media. Thallium-cation uptake has been observed only from the latter and its extent was found to be a function of electrode potential and composition. At potentials near 0.0 V (RHE), the amount of adsorbed Tl+ exhibited a maximum, and decreased with increasing potential, reaching a broad minimum in the range 0.4–0.8 V. A further increase in the electrode potential, above about 1.0 V, led to an increase of adsorbed thallium species, essentially due to deposition of a few layers of Tl(III) hydroxide. In fact, the release of the latter species was found to be much slower than that of thallium ions adsorbed at 0.0 V. For the latter, in turn, the double injection/ejection mechanism, currently accepted to explain the charge-storage in oxide electrodes, seems to be confirmed. The high Γ values attained at 0.0 V indicate that the large ionic radius of Tl+ does not prevent its diffusion through the thinner pore texture of the oxide coatings, possibly because of its poor hydration, related with lower charge density at the ion surfaces.




Similar content being viewed by others
References
Trasatti S, Lodi G (1981) In: Trasatti S (ed) Electrodes of conductive metal oxides, part B. Elsevier, Amsterdam, p 521
Nidola A (1981) In: Trasatti S (ed) Electrodes of conductive metal oxides, part B. Elsevier, Amsterdam, p 627
Novak DM, Tilak BV, Conway BE (1982) In: Conway BE, Bockris JO’M (eds) Modern aspects of electrochemistry, vol. 14. New York, Plenum, p 195
Trasatti S (1998) Chimica e l’Ind 80:199
Trasatti S, Kurzweil P (1994) Platinum Metals Rev 38:46
Conway BE (1999) Electrochemical supercapacitors, scientific fundamentals and technological applications. Kluwer Academics/Plenum Publishers, New York, ch 11
Trasatti S, Lodi G (1980) In: Trasatti S (ed) Electrodes of conductive metal oxides, Part A. Elsevier, Amsterdam, 198, p 301
Trasatti S (1987) Electrochim Acta 32:369
Trasatti S (1984) Electrochim Acta 29:1503
Parks GA (1965) Chem Rev 65:177
Trasatti S (1980) J Electroanal Chem 111:125
Ardizzone S, Siviglia P, Trasatti S (1981) J Electroanal Chem 122:395
Ahmed SM (1972) In: Diggle JW (ed) Oxides and oxide films, vol. 1. New York, Marcel Dekker, p 319
Ardizzone S, Trasatti S (1996) Adv Colloid Interface Sci 64:173
Kazarinov VE, Andreev VN (1977) Elektrokhimiya 13:585
Kazarinov VE, Andreev VN (1978) Elektrokhimiya 14:577
Andreev VN, Kazarinov VE, Kokoulina DV, Krishtalik LI (1978) Elektrokhimiya 14:1278
Andreev VN, Kokoulina DV, Krasovitskaya YuI, Kazarinov VE, Krishtalik LI (1978) Dvoinoi Sloi i Adsorbtsiya na Tverdykh Elektrodakh 5:13
Kazarinov VE, Krishtalik LI, Pleskov Yu.V (1977) Trans SAEST 12:287
Andreev VN, Kokoulina DV (1978) Elektrokhimiya 15:1417
Mayorov AP, Andreev VN, Kazarinov VE (1980) Elektrokhimiya 16:124
Kazarinov VE, Andreev VN (1978) Proceeding of the 3rd Japan-SSSR seminar on electro-chemistry. Electrode process and its new approach chemical energy problem, vol. 2, Kyoto, Japan, Nov. 27–Dec. 2, 1978, p 318
Andreev VN, Balashova NA, Kazarinov VE (1978) Extended abstracts 29th meeting international society of electrochemistry, 1978, Part 1, p 382
Kazarinov VE, Andreev VN, Mayorov AP (1981) J Electroanal Chem 130:227
Andreev VN, Kazarinov VE Adsorption properties of oxide ruthenium-titanium oxide electrodes. In “Itogi Nauki i Tekhn.”, Ser. Electrochemistry, VINITI, Moscow, 1983, p 47
Robinson RA, Stokes RH (1968) Electrolyte solutions. Butterworths, London, p 408
Kazarinov VE (1966) Elektrokhimiya 2:1120
Burke LD, Murphy OJ (1979) J Electroanal Chem 96:19
Burke LD, Whelan DP (1979) J Electroanal Chem 103:179
Guerrini E, Consonni V, Trasatti S (2005) J Solid State Electrochem 9:320
Ardizzone S, Fregonara G, Transatti S (1990) Electrochimica Acta 35:263
Burke LD, Murphy JO (1980) J Electroanal Chem 109:199
De Battisti A, Lodi G, Cappadonia M, Battaglin G, Kotz R (1989) J Electrochem Soc 136:2596
McIntyre JDE, Basu S, Peck WF, Brown WL, Augustyniak WM (1982) Phys Rev B 25:7242
Amadelli R, Markovic N, Adzic R, Yeager E (1983) J Electroanal Chem 159:391
Weast RC (ed) (1993–1994) Handbook of Chemistry and Physics. CRC Press, 74th edn, section 8, p 25
Battaglin G, De Battisti A, Barbieri A, Giatti A, Marchi A (1991) Surf Sci 251:73
De Battisti A, Barbieri A, Giatti A, Battaglin G, Daolio S, Boscolo Boscoletto A (1991) J Mater Chem 1:191
Daolio S, Facchin B, Pagura C, De Battisti A, Barbieri A, Kristof J (1994) J Mater Chem 4:1255
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferro, S., Donatoni, M., De Battisti, A. et al. Adsorption of thallium cations on RuO2–TiO2 electrodes. J Appl Electrochem 37, 1389–1394 (2007). https://doi.org/10.1007/s10800-007-9417-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-007-9417-y