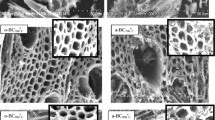
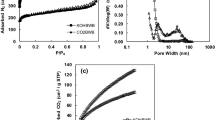
Abstract
Biochar, a by-product of woody biomass pyrolysis, is investigated as a renewable and low-cost carbon-based electrode material for electric double layer (EDL) applications. To increase the surface area and porosity of the biochar chemical (7 M KOH) and thermal (at 675 and 1,000 °C, respectively) activation treatments are applied. The thermo-chemically activated biochar samples are investigated by a combination of physico-chemical surface characterization and electrochemical methods to reveal the relationship between the activation process variables, the resulting porous carbon structural features and EDL capacitance. For electrochemical testing, the activated biochar is sprayed onto Ni mesh current collectors with or without Nafion® as binder. Based on cyclic voltammetry experiments in 0.1 M NaCl–0.1 M NaOH a maximum EDL capacitance of 167 F g−1 is obtained for the activated biochar electrode prepared at 675 °C. The latter capacitance is about 50 times higher than the EDL capacitance of a Vulcan XC-72 electrode prepared and tested under identical conditions. The activated biochar electrodes show also promising galvanostatic charge/discharge behavior and electrical conductivities up to 0.058 S cm−1 indicating suitability for EDL-type applications.











Similar content being viewed by others
References
Yang K-L, Ying T-Y, Yiacoumi S et al (2001) Electrosorption of ions from aqueous solutions by carbon aerogel: an electrical double-layer model. Langmuir 17:1961–1969. doi:10.1021/la001527s
Wang G, Qian B, Dong Q et al (2013) Highly mesoporous activated carbon electrode for capacitive deionization. Sep Purif Technol 103:216–221. doi:10.1016/j.seppur.2012.10.041
Ghosh A, Lee YH (2012) Carbon-based electrochemical capacitors. ChemSusChem 5:480–499. doi:10.1002/cssc.201100645
Hulicova-Jurcakova D, Fiset E, Lu GQM, Bandosz TJ (2012) Changes in surface chemistry of carbon materials upon electrochemical measurements and their effects on capacitance in acidic and neutral electrolytes. ChemSusChem 5:2188–2199. doi:10.1002/cssc.201200376
Foo KY, Hameed BH (2009) A short review of activated carbon assisted electrosorption process: an overview, current stage and future prospects. J Hazard Mater 170:552–559. doi:10.1016/j.jhazmat.2009.05.057
Oren Y (2008) Capacitive deionization (CDI) for desalination and water treatment—past, present and future (a review). Desalination 228:10–29. doi:10.1016/j.desal.2007.08.005
Frackowiak E, Béguin F (2001) Carbon materials for the electrochemical storage of energy in capacitors. Carbon 39:937–950. doi:10.1016/S0008-6223(00)00183-4
Salitra G, Soffer A, Eliad L et al (2000) Carbon electrodes for double-layer capacitors I. Relations between ion and pore dimensions. J Electrochem Soc 147:2486–2493. doi:10.1149/1.1393557
Noked M, Avraham E, Soffer A, Aurbach D (2009) The rate-determining step of electroadsorption processes into nanoporous carbon electrodes related to water desalination. J Phys Chem C 113:21319–21327. doi:10.1021/jp905987j
Gao Y, Pan L, Li H et al (2009) Electrosorption behavior of cations with carbon nanotubes and carbon nanofibres composite film electrodes. Thin Solid Films 517:1616–1619. doi:10.1016/j.tsf.2008.09.065
Dai H, Wong EW, Lieber CM (1996) Probing electrical transport in nanomaterials: conductivity of individual carbon nanotubes. Science 272:523–526. doi:10.1126/science.272.5261.523
Kim YJ, Yang C-M, Park KC et al (2012) Edge-enriched, porous carbon-based, high energy density supercapacitors for hybrid electric vehicles. ChemSusChem 5:535–541. doi:10.1002/cssc.201100511
Mitra S, Sampath S (2004) Electrochemical Capacitors Based on Exfoliated Graphite Electrodes. Electrochem Solid State Lett 7:A264–A268. doi:10.1149/1.1773752
Farma R, Deraman M, Awitdrus A et al (2013) Preparation of highly porous binderless activated carbon electrodes from fibres of oil palm empty fruit bunches for application in supercapacitors. Bioresour Technol 132:254–261. doi:10.1016/j.biortech.2013.01.044
Farmer JC, Bahowick SM, Harrar JE et al (1997) Electrosorption of chromium ions on carbon aerogel electrodes as a means of remediating ground water. Energy Fuels 11:337–347. doi:10.1021/ef9601374
Seo S-J, Jeon H, Lee JK et al (2010) Investigation on removal of hardness ions by capacitive deionization (CDI) for water softening applications. Water Res 44:2267–2275. doi:10.1016/j.watres.2009.10.020
Ayranci E, Conway BE (2001) Adsorption and electrosorption of ethyl xanthate and thiocyanate anions at high-area carbon-cloth electrodes studied by in situ UV spectroscopy: development of procedures for wastewater purification. Anal Chem 73:1181–1189. doi:10.1021/ac000736e
Yang C-M, Choi W-H, Na B-K et al (2005) Capacitive deionization of NaCl solution with carbon aerogel-silicagel composite electrodes. Desalination 174:125–133. doi:10.1016/j.desal.2004.09.006
Corry B (2008) Designing carbon nanotube membranes for efficient water desalination. J Phys Chem B 112:1427–1434. doi:10.1021/jp709845u
Oren Y, Soffer A (1978) Electrochemical parametric pumping. J Electrochem Soc 125:869–875. doi:10.1149/1.2131570
Gabelich CJ, Tran TD, Suffet IH “Mel” (2002) Electrosorption of inorganic salts from aqueous solution using carbon aerogels. Environ Sci Technol 36:3010–3019. doi: 10.1021/es0112745
Ban A, Schafer A, Wendt H (1998) Fundamentals of electrosorption on activated carbon for wastewater treatment of industrial effluents. J Appl Electrochem 157:602–615. doi:10.1023/A:1003247229049
Dai K, Shi L, Fang J et al (2005) NaCl adsorption in multi-walled carbon nanotubes. Mater Lett 59:1989–1992. doi:10.1016/j.matlet.2005.01.042
Farmer JC, Mack GV, Fix DV (1996) The Use of Carbon Aerogel Electrodes for Deionizing Water and Treating Aqueous Process Wastes. P 4th Int Congr Environ Conscious Des Manuf
Zou L, Morris G, Qi D (2008) Using activated carbon electrode in electrosorptive deionisation of brackish water. Desalination 225:329–340. doi:10.1016/j.desal.2007.07.014
Ghodbane I, Hamdaoui O (2008) Removal of mercury(II) from aqueous media using eucalyptus bark: kinetic and equilibrium studies. J Hazard Mater 160:301–309. doi:10.1016/j.jhazmat.2008.02.116
Alvi F, Ram MK, Basnayaka PA et al (2011) Graphene–polyethylenedioxythiophene conducting polymer nanocomposite based supercapacitor. Electrochim Acta 56:9406–9412. doi:10.1016/j.electacta.2011.08.024
Lehmann J, Joseph S (2009) Biochar for environmental management. Earthscan, London
Marsh H, Heintz EA, Rodríguez-Reinoso F (1997) Introduction to carbon technologies. University of Alicante Publications, Alicante
Azargohar R, Dalai AK (2008) Steam and KOH activation of biochar: experimental and modeling studies. Microporous Mesoporous Mater 110:413–421. doi:10.1016/j.micromeso.2007.06.047
Panić VV, Stevanović RM, Jovanović VM, Dekanski AB (2008) Electrochemical and capacitive properties of thin-layer carbon black electrodes. J Power Sources 181:186–192. doi:10.1016/j.jpowsour.2008.03.048
Bansal RC, Goyal M (2005) Activated carbon adsorption. CRC Press, Boca Raton
Patrick JW (1995) Porosity in carbons: characterization and applications. Halsted Press, Sydney
Franklin RE (1951) Crystallite growth in graphitizing and non-graphitizing carbons. Proc Roy Soc Lond Ser A 209:196–218
Yu JT, Dehkhoda AM, Ellis N (2011) Development of biochar-based catalyst for transesterification of canola oil. Energy Fuels 25:337–344. doi:10.1021/ef100977d
Brewer CE, Schmidt-Rohr K, Satrio JA, Brown RC (2009) Characterization of biochar from fast pyrolysis and gasification systems. Environ Prog Sustain Energy 28:386–396. doi:10.1002/ep.10378
Sun K, Ro K, Guo M et al (2011) Sorption of bisphenol A, 17α-ethinyl estradiol and phenanthrene on thermally and hydrothermally produced biochars. Bioresour Technol 102:5757–5763. doi:10.1016/j.biortech.2011.03.038
Singh B, Singh BP, Cowie AL (2010) Characterisation and evaluation of biochars for their application as a soil amendment. Aust J Soil Res 48:516–525
Azargohar R, Dalai A (2006) Biochar as a precursor of activated carbon. Appl Biochem Biotech 131:762–773. doi:10.1385/ABAB:131:1:762
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 44:1247–1253. doi:10.1021/es9031419
Celzard A, Mareche J, Payot F, Furdin G (2002) Electrical conductivity of carbonaceous powders. Carbon 40:2801–2815. doi:10.1016/S0008-6223(02)00196-3
Pandolfo AG, Hollenkamp AF (2006) Carbon properties and their role in supercapacitors. J Power Sources 157:11–27. doi:16/j.jpowsour.2006.02.065
Wei ZD, Yan C, Tan Y et al (2008) Spontaneous reduction of Pt(IV) onto the sidewalls of functionalized multiwalled carbon nanotubes as catalysts for oxygen reduction reaction in PEMFCs. J Phys Chem C 112:2671–2677. doi:10.1021/jp709936p
Hsu Y-K, Chen Y-C, Lin Y-G et al (2012) High-cell-voltage supercapacitor of carbon nanotube/carbon cloth operating in neutral aqueous solution. J Mater Chem 22:3383. doi:10.1039/c1jm14716a
Wang G, Ling Y, Qian F et al (2011) Enhanced capacitance in partially exfoliated multi-walled carbon nanotubes. J Power Sources 196:5209–5214. doi:10.1016/j.jpowsour.2011.02.019
Bao L, Zang J, Li X (2011) Flexible Zn2SnO4/MnO2 core/shell nanocable−carbon microfiber hybrid composites for high-performance supercapacitor electrodes. Nano Lett 11:1215–1220. doi:10.1021/nl104205s
Zhao Y, Zheng M, Cao J et al (2008) Easy synthesis of ordered meso/macroporous carbon monolith for use as electrode in electrochemical capacitors. Mater Lett 62:548–551. doi:16/j.matlet.2007.06.002
Honda Y, Haramoto T, Takeshige M et al (2007) Aligned MWCNT sheet electrodes prepared by transfer methodology providing high-power capacitor performance. Electrochem Solid State Lett 10:A106–A110. doi:10.1149/1.2437665
Miller JM, Dunn B, Tran TD, Pekala RW (1997) Deposition of ruthenium nanoparticles on carbon aerogels for high energy density supercapacitor electrodes. J Electrochem Soc 144:L309–L311. doi:10.1149/1.1838142
Acknowledgments
The authors acknowledge the generous financial support of the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery and Discovery Accelerator Supplement Grant program and Rio Tinto Alcan Inc. in the form of the Graduate Student Award to Amir Mehdi Dehkhoda.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dehkhoda, A.M., Ellis, N. & Gyenge, E. Electrosorption on activated biochar: effect of thermo-chemical activation treatment on the electric double layer capacitance. J Appl Electrochem 44, 141–157 (2014). https://doi.org/10.1007/s10800-013-0616-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-013-0616-4