Abstract
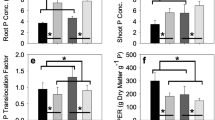
The characterization of nutrient and biostimulant effects in crops is complex and needs rigorous evaluations. In this study, we evaluated morphological and molecular responses induced by microalgae (Chlorella vulgaris and Scenedesmus quadricauda) extracts in Beta vulgaris L. The two microalgae extracts were firstly characterized by CNS, Fourier transform infrared spectroscopic analysis (FT-IR), and carbon-13 nuclear magnetic resonance (13C NMR). Seedlings were grown in Hoagland’s solution under controlled conditions. After 5 days of growth, 2 mL L−1 (1 mg Corg L−1) and 4 mL L−1 (2 mg Corg L−1) of the two microalgae extracts were added to the Hoagland solution. Roots were sampled 36 h after treatments. Inductively coupled plasma spectrometry (ICP-OES) and nanofluidic real-time PCR (OpenArray system) were used for sample profiling. Fifty-three sugar beet genes putatively involved in sulfate starvation were tested in treated and untreated samples. Root morphological traits were measured by means of a scanner-based image analysis system. Multivariate statistical analysis revealed no significant changes in the ionomic profile of Hoagland’s solutions treated with the two microalgae extracts with respect to that of the untreated solution. At the molecular level, microalgae extract supplies upregulated many of the evaluated genes. Functional categorization revealed these genes to be related to various biological pathways and processes including primary and secondary metabolism and intracellular transport. At the morphological level, the treated seedlings showed significantly higher values for root traits related to soil exploration and nutrient uptake, such as total root length, fine root length (diameter < 0.5 mm), and number of root tips, than the untreated plants. These data indicate that microalgae extracts have biostimulant effects on the expression of root traits and genes related to nutrient acquisition in sugar beet.






Similar content being viewed by others
References
Akhter M, Majumdar RD, Fortier-McGill B, Soong R, Liaghati-Mobarhan Y, Simpson M, Arhonditsis G, Schmidt S, Heumann H, Simpson AJ (2016) Identification of aquatically available carbon from algae through solution-state NMR of whole 13C-labelled cells. Anal Bioanal Chem 408:4357–4370
Arnon DI, Hoagland DR (1940) Crop production in artificial culture solution and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci 50:463–483
Baglieri A, Gennari M, Ioppolo A, Leinweber P, Nègre M (2012) Comparison between the humic acids characteristics of two andisols of different age by: FT-IR and 1 H-NMR spectroscopy and py-FIMS. Geochem Int 50:148–158
Baglieri A, Cadili V, Monterumici CM, Gennari M, Tabasso S, Montoneri E, Nardi S, Negre M (2014) Fertilization of bean plants with tomato plants hydrolysates. Effect on biomass production, chlorophyll content and N assimilation. Sci Hortic 176:194–199
Baglieri A, Sidella S, Barone V, Fragalà F, Silkina A, Nègre M, Gennari M (2016) Cultivating Chlorella vulgaris and Scenedesmus quadricauda microalgae to degrade inorganic compounds and pesticides in water. Environ Sci Pollut Res 23:18165–18174
Beijerinck MW (1890) Culturversuche mit Zoochlorellen, Lichenengonidien und anderen niederen Algen. Bot Ztg 48:1–23
Biancardi E, McGrath JM, Panella LW, Lewellen RT, Stevanato P (2010) Sugar beet. In: Bradshaw J (ed) Handbook of plant breeding, Tuber and Root Crops, vol 4. Springer, New York, pp 173–219
Billard V, Etienne P, Jannin L, Garnica M, Cruz F, Garcia-Mina JM, Yvin JC, Ourry A (2014) Two biostimulants derived from algae or humic acid induce similar responses in the mineral content and gene expression of winter oilseed rape (Brassica napus L.) J Plant Growth Regul 33:305–316
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Bulgari R, Cocetta G, Trivellini A, Vernieri P, Ferrante A (2015) Biostimulants and crop responses: a review. Biol Agric Hortic 31:1–17
Bumbak F, Cook S, Zachleder V, Hauser S, Kovar K (2011) Best practices in heterotrophic high-cell-density microalgal processes: achievements, potential and possible limitations. Appl Microbiol Biotechnol 91:31
Chen SK, Subler S, Edwards CA (2002) Effects of agricultural biostimulants on soil microbial activity and nitrogen dynamics. Appl Soil Ecol 19:249–260
Coppens J, Lindeboom R, Muys M, Coessens W, Alloul A, Meerbergen K, Lievens B, Clauwaert P, Boon N, Vlaeminck SE (2016) Nitrification and microalgae cultivation for two-stage biological nutrient valorization from source separated urine. Bioresour Technol 211:41–50
Crofcheck C, Xinyi E, Shea A, Montross M, Crocker M, Andrews R (2012) Influence of media composition on the growth rate of Chlorella vulgaris and Scenedesmus acutus utilized for CO2 mitigation. J Biochem Technol 4:589–594
Devi MP, Subhash GV, Mohan SV (2012) Heterotrophic cultivation of mixed microalgae for lipid accumulation and wastewater treatment during sequential growth and starvation phases: effect of nutrient supplementation. Renew Energy 43:276–283
Di Caprio F, Altimari P, Pagnanelli F (2015) Integrated biomass production and biodegradation of olive mill wastewater by cultivation of Scenedesmus sp. Algal Res 9:306–311
du Jardin P (2015) Plant biostimulants: definition, concept, main categories and regulation. Sci Hortic 196:3–14
Duygu DY, Udoh AU, Ozer TB, Akbulut A, Erkaya IA, Yildiz K, Guler D (2012) Fourier transform infrared (FTIR) spectroscopy for identification of Chlorella vulgaris Beijerinck 1890 and Scenedesmus obliquus (Turpin) Kützing 1833. Afr J Biotechnol 11:3817–3824
Faheed FA, Abd-El Fattah Z (2008) Effect of Chlorella vulgaris as bio-fertilizer on growth parameters and metabolic aspects of lettuce plant. J Agric Soc Sci 4:165–169
Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S (2004) Architecture of the photosynthetic oxygen-evolving center. Science 303:1831–1838
Fierro S, del Pilar S-SM, Copalcua C (2008) Nitrate and phosphate removal by chitosan immobilized Scenedesmus. Bioresour Technol 99:1274–1279
Garcia-Gonzalez J, Sommerfeld M (2016) Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J Appl Phycol 28:1051–1061
Huang CZ, Wang SL, Chen L, Lemieux C, Otis C, Turmel M, Liu XQ (1994) The Chlamydomonas chloroplast clpP gene contains translated large insertion sequences and is essential for cell growth. Mol Gen Genet 244:151–159
Jones PD, Lister DH, Jaggard KW, Pidgeon JD (2003) Future climate impact on the productivity of sugar beet (Beta vulgaris L.) in Europe. Clim Chang 58:93–108
Köhler HR, Triebskorn R (2013) Wildlife ecotoxicology of pesticides: can we track effects to the population level and beyond? Science 341:759–765
Lopez CVG, Garcia MDCC, Fernandez FGA, Bustos CS, Chisti Y, Sevilla JMF (2010) Protein measurements of microalgal and cynobacterial biomass. Biores Technol 101:7587–7591
Lucini L, Rouphael Y, Cardarelli M, Canaguier R, Kumar P, Colla G (2015) The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci Hortic 182:124–133
Makarevičienė V, Skorupskaitė V, Andrulevičiūtė V (2012) Biomass and oil production of green microalgae Scenedesmus sp. using different nutrients and growth. Environ Res Eng Manag 62:5–13
Mann JE, Myers J (1968) On pigments, growth and photosynthesis of Phaedactylum tricornutum. J Phycol 4:349–355
Metting B, Zimmerman WJ, Crouch IJ, van Staden J (1990) Agronomic uses of seaweed and microalgae. In: Akatsuka I (ed) Introduction to applied phycology. SPB Academic Publishing, The Hague, pp 269–307
Miller RH (1990) Soil microbiological inputs for sustainable agricultural systems. In: Edwards CA, Lal R, Madden P, Miller RH, House G (eds) Sustainable agricultural systems. Soil and Water Conservation Society, Ankeny, pp 614–623
Nabti E, Jha B, Hartmann A (2016) Impact of seaweeds on agricultural crop production as biofertilizer. Int J Environ Sci Technol 1–16
Norton TA, Melkonian M, Andersen RA (1996) Algal biodiversity. Phycologia 35:308–326
Olofsson M, Lamela T, Nilsson E, Bergé JP, Del Pino V, Uronen P, Legrand C (2012) Seasonal variation of lipids and fatty acids of the microalgae Nannochloropsis oculata grown in outdoor large-scale photobioreactors. Energies 5:1577–1592
Rocha GS, Pinto FHV, Melão MGG, Lombardi AT (2015) Growing Scenedesmus quadricauda in used culture media: is it viable? J Appl Phycol 27:171–178
Safi C, Ursu AV, Laroche C, Zebib B, Merah O, Pontalier PY, Vaca-Garcia C (2014) Aqueous extraction of proteins from microalgae: effect of different cell disruption methods. Algal Res 3:61–65
Sanudo-Wilhelmy SA, Gómez-Consarnau L, Suffridge C, Webb EA (2014) The role of B vitamins in marine biogeochemistry. Annu Rev Mar Sci 6:339–367
Shaaban MM (2001) Green microalgae water extract as foliar feeding to wheat plants. Pak J Biol Sci 4:628–632
Sigee DC, Dean A, Levado E, Tobin MJ (2002) Fourier-transform infrared spectroscopy of Pediastrum duplex: characterization of a micro-population isolated from a eutrophic lake. Eur J Phycol 37:19–26
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bact Rev 35:171
Stevanato P, Trebbi D, Saccomani M (2010) Root traits and yield in sugar beet: identification of AFLP markers associated with root elongation rate. Euphytica 173:289–298
Stevanato P, Trebbi D, Bertaggia M, Colombo M, Broccanello C, Concheri G, Saccomani M (2011) Root traits and competitiveness against weeds in sugar beet. Int Sugar J 113:497–501
Stevanato P, Fedito P, Trebbi D, Cagnin M, Saccomani M, Cacco G (2015) Effect of sulfate availability on root traits and microRNA395 expression in sugar beet. Biol Plantarum 59:491–496
Vigani M, Parisi C, Rodríguez-Cerezo E, Barbosa MJ, Sijtsma L, Ploeg M, Enzing C (2015) Food and feed products from micro-algae: market opportunities and challenges for the EU. Trends Food Sci Technol 42:81–92
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(XLSX 53 kb)
Rights and permissions
About this article
Cite this article
Barone, V., Baglieri, A., Stevanato, P. et al. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.). J Appl Phycol 30, 1061–1071 (2018). https://doi.org/10.1007/s10811-017-1283-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1283-3